Abstract
Study Design
This study utilized male Wistar rats to investigate the effects of time-restricted feeding (TRF) on high-fat diet (HFD)-induced alterations in neuron-glial interactions and gene expression levels in the spinal cord (T5-T9).
Objectives
To evaluate whether TRF mitigates HFD-induced alterations in microglial morphology, astrocyte numbers, perineuronal net (PNN) integrity, purinergic receptor expression, inflammation and circadian rhythm-related gene expression in the spinal cord.
Setting
Amsterdam University Medical Centers, location AMC, The Netherlands.
Methods
Male Wistar rats were initially fed either a standard chow diet or a HFD ad libitum for 4 weeks. After this period, rats in the HFD group were further divided into two subgroups: continued HFD ad libitum or HFD with TRF for an additional 4 weeks. Rats in the chow group continued with ad libitum feeding throughout the experimental period. At the end of the intervention, spinal cords (T5–T9) were collected for analysis. Microglial morphology, astrocyte cell numbers, and PNN integrity were assessed in the spinal cord. Expression levels of purinergic receptors, inflammation and clock genes were analyzed to investigate neuron-glial interactions and circadian rhythm stabilization.
Results
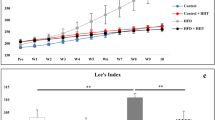
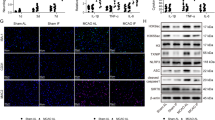
TRF reduced microglial activation, preserved PNN integrity, suppressed HFD-induced upregulation of purinergic receptors, and stabilized circadian clock gene expression.
Conclusions
These findings suggest that TRF is a promising non-pharmacological strategy to counteract obesogenic diet-induced perineuronal net degradation and neuroinflammation, highlighting its potential as a lifestyle-based intervention for pain management.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data used in the current study are available from the corresponding author upon reasonable request.
References
Eichwald T, Talbot S. Neuro-immunity controls obesity-induced pain. Front Hum Neurosci. 2020;14:181.
Zdziarski LA, Wasser JG, Vincent HK. Chronic pain management in the obese patient: a focused review of key challenges and potential exercise solutions. J Pain Res. 2015;8:63–77.
Liang Y-J, Feng S-Y, Qi Y-P, Li K, Jin Z-R, Jing H-B, et al. Contribution of microglial reaction to increased nociceptive responses in high-fat-diet (HFD)-induced obesity in male mice. Brain Behav Immun. 2019;80:777–92.
Tramullas M, Finger BC, Dinan TG, Cryan JF. Obesity takes its toll on visceral pain: high-fat diet induces toll-like receptor 4-dependent visceral hypersensitivity. PLoS ONE. 2016;11:e0155367.
Malcangio M. Role of the immune system in neuropathic pain. Scand J Pain. 2019;20:33–7.
Bjørklund G, Aaseth J, Doşa MD, Pivina L, Dadar M, Pen JJ, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153–65.
Cuevas-Cervera M, Perez-Montilla JJ, Gonzalez-Muñoz A, Garcia-Rios MC, Navarro-Ledesma S. The effectiveness of intermittent fasting, time restricted feeding, caloric restriction, a ketogenic diet and the mediterranean diet as part of the treatment plan to improve health and chronic musculoskeletal pain: a systematic review. Int J Environ Res Public Health. 2022;19:6698.
Vergne-Salle P, Bertin P. Chronic pain and neuroinflammation. Jt Bone Spine. 2021;88:105222.
Manoogian EN, Chow LS, Taub PR, Laferrère B, Panda S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr Rev. 2022;43:405–36.
Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23:1048–59.
Ellis A, Bennett D. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37.
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, et al. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–64.
Thawkar BS, Kaur G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J Neuroimmunol. 2019;326:62–74.
Illes P. P2X7 receptors amplify CNS damage in neurodegenerative diseases. Int J Mol Sci. 2020;21:5996.
Jin XH, Wang LN, Zuo JL, Yang JP, Liu SL. P2X4 receptor in the dorsal horn partially contributes to brain-derived neurotrophic factor oversecretion and toll-like receptor-4 receptor activation associated with bone cancer pain. J Neurosci Res. 2014;92:1690–702.
Tajerian M, Clark JD. The role of the extracellular matrix in chronic pain following injury. Pain. 2015;156:366–70.
Tansley S, Gu N, Guzmán AU, Cai W, Wong C, Lister KC, et al. Microglia-mediated degradation of perineuronal nets promotes pain. Science. 2022;377:80–6.
Liu J, Wong SSC. Molecular mechanisms and pathophysiological pathways of high-fat diets and caloric restriction dietary patterns on pain. Anesth Analg. 2023;137:137–52.
Richner M, Jager SB, Siupka P, Vaegter CB. Hydraulic Extrusion of the Spinal Cord and Isolation of Dorsal Root Ganglia in Rodents. J Vis Exp. 2017;55226. https://doi.org/10.3791/55226.
Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20:451–65.
Lenz FA. The human pain system: experimental and clinical perspectives. Cambridge University Press; 2010.
Sapio MR, Vazquez FA, Loydpierson AJ, Maric D, Kim JJ, LaPaglia DM, et al. Comparative analysis of dorsal root, nodose and sympathetic ganglia for the development of new analgesics. Front Neurosci. 2020;14:615362.
Vidal-Itriago A, Radford RAW, Aramideh JA, Maurel C, Scherer NM, Don EK, et al. Microglia morphophysiological diversity and its implications for the CNS. Front Immunol. 2022;13:997786.
Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–31.
Wang H, Zhang Y, Ma X, Wang W, Xu X, Huang M, et al. Spinal TLR4/P2X7 receptor-dependent NLRP3 inflammasome activation contributes to the development of tolerance to morphine-induced antinociception. J Inflamm Res. 2020;13:571–82.
Tozaki-Saitoh H, Takeda H, Inoue K. The role of microglial purinergic receptors in pain signaling. Molecules. 2022;27:1919.
Zhang Y, Yin HY, Rubini P, Tang Y, Illes P. A possible causal involvement of neuroinflammatory, purinergic P2X7 receptors in psychiatric disorders. Curr Neuropharmacol. 2022;20:2142–55.
Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. Eur J Neurosci. 2013;37:1350–6.
Blancas-Velazquez AS, Unmehopa UA, Eggels L, Koekkoek L, Kalsbeek A, Mendoza J, et al. A free-choice high-fat high-sugar diet alters day-night Per2 gene expression in reward-related brain areas in rats. Front Endocrinol. 2018;9:154.
Chiang C-Y, Sessle B, Dostrovsky J. Role of astrocytes in pain. Neurochem Res. 2012;37:2419–31.
Gaudet AD, Fonken LK, Ayala MT, Bateman EM, Schleicher WE, Smith EJ, et al. Spinal cord injury in rats disrupts the circadian system. eNeuro, 2018;5:ENEURO.0328-18.2018.
Ye Y, Xu H, Xie Z, Wang L, Sun Y, Yang H, et al. Time-restricted feeding reduces the detrimental effects of a high-fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front Nutr. 2020;7:596285.
de Moura EDM, Dos Reis SA, da Conceicao LL, Sediyama C, Pereira SS, de Oliveira LL, et al. Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetol Metab Syndr. 2021;13:32.
Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14:140–62.
Alvord VM, Pendergast JS. The estrous cycle coordinates the circadian rhythm of eating behavior in mice. J Biol Rhythm. 2024;39:413–22.
Mermet J, Yeung J, Naef F. Systems chronobiology: global analysis of gene regulation in a 24-hour periodic world. Cold Spring Harb Perspect Biol. 2017;9:a028720.
Acknowledgements
We sincerely thank Nikita Korpel (Department of Population Health Sciences, Institute for Risk Assessment Sciences, Utrecht University) and Ewout Foppen (Endocrinology Lab, Amsterdam UMC) for their invaluable support with the animal study and technical assistance.
Funding
This study was sponsored by the Netherlands-Canada Type 2 Diabetes Research Consortium (ZonMW 459001021).
Author information
Authors and Affiliations
Contributions
H.J. and C.Y. set up the experiments. J.J., M.D. and H.J. performed all the animal housing and time-restricted feeding experiments. H.J., D.P., and M.D. performed spinal cord tissue collection experiment. H.J., M.D., and S.W. performed immunohistochemistry experiment. H.J. performed RNA isolation and PCRs experiments. H.J. and C.Y. conceived the idea and the experimental design. H.J., A.K., and C.Y. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiao, H., Jermei, J., Poormoghadam, D. et al. Time-restricted feeding modulates neuron-glial interactions and circadian rhythm in the spinal cord of male Wistar rats fed a high-fat diet. Spinal Cord 63, 437–443 (2025). https://doi.org/10.1038/s41393-025-01106-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41393-025-01106-9