Abstract
Study Design
Animal study.
Objectives
The NLRP3 inflammasome is a key mediator in secondary cascade of spinal cord injury (SCI), making it a potential therapeutic target. This study aimed to develop a nanoformulated version of fingolimod and investigate its effects on neuroinflammation, NLRP3 inflammasome activity, glial activation, lesion volume, and functional outcomes in a rat model of moderate contusive SCI.
Setting
Experimental animal research laboratory, AlSafwa University College, Karbala, Iraq.
Methods
Fingolimod-loaded PLGA nanoparticles were synthesized using the emulsion solvent evaporation method and characterized for size, morphology, and drug release. Adult male Wistar rats underwent a standardized contusion SCI at T9 and were randomized into three groups (n = 13): Sham, SCI + Vehicle, and SCI + Nano-Fingolimod (10 ng/ml). Behavioral assessments using the BBB locomotor test were performed on days 1, 3, 5, 7, 10, 12, and 14 post-injuries. Histological analysis quantified lesion volume and inflammatory cell infiltration. Immunofluorescence (IF) and Western blotting were used to evaluate the expression of NLRP3, ASC, cleaved caspase-1, IL-1β, GFAP, and Iba-1.
Results
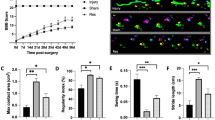
Nano-fingolimod significantly improved BBB locomotor scores compared to the SCI group (p < 0.0001), indicating enhanced motor recovery. Histological examination revealed reduced lesion volume and inflammatory cell density in the treatment group. IF and Western blot analyses showed marked suppression of NLRP3 inflammasome signaling components (NLRP3, ASC, cleaved caspase-1, and IL-1β) and reduced glial activation (GFAP, Iba-1).
Conclusion
Nanoformulated fingolimod provides neuroprotection and improves functional recovery after SCI by attenuating NLRP3 inflammasome activation and glial reactivity. Targeted nanodelivery enhances its CNS bioavailability and reduces toxicity, positioning it as a promising candidate for SCI therapy.
Sponsorship
None.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated analysed during the current study are available from the corresponding author on reasonable request.
References
Norimatsu Y, Ohmori T, Kimura A, Madoiwa S, Mimuro J, Seichi A, et al. FTY720 improves functional recovery after spinal cord injury by primarily nonimmunomodulatory mechanisms. Am J Pathol. 2012;180:1625–35.
Lee KD, Chow WN, Sato-Bigbee C, Graf MR, Graham RS, Colello RJ, et al. FTY720 reduces inflammation and promotes functional recovery after spinal cord injury. J Neurotrauma. 2009;26:2335–44.
Chen Y, Liu R, Cai W, Jiang L, Chen K, Shi J, et al. Davunetide promotes structural and functional recovery of the injured spinal cord by promoting autophagy. Neural Regen Res. 2025;1:10–4103.
Zhang H, He K, Zhao Y, Peng Y, Feng D, Wang J, et al. fNIRS biomarkers for stratifying poststroke cognitive impairment: Evidence from frontal and temporal cortex activation. Stroke. 2025;56:3245–56.
Xu G, Huo C, Yin J, Zhong Y, Sun G, Fan Y, et al. Test-retest reliability of fNIRS in resting-state cortical activity and brain network assessment in stroke patients. Biomed Opt Express. 2023;14:4217–36.
Shirzad S, Riyahi Rad M, Rezaei M, Tayaranian Marvian M, Abroumand Gholami A, Forouzanfar F, et al. Effect of pretreatment with Devil’s Claw on locomotor activity, infarct volume, and neuronal density in focal cerebral ischemia in rats. Avicenna J Phytomedicine. 2024;14:485–95.
Forghani N, Hosseinian S, Akhoond-Ali Z, Gholami AA, Assaran-Darban R, Vafaee F. Effect of acute and chronic stress on memory impairment and hippocampal oxidative stress following global cerebral ischemia in adult male rats. Res Pharm Sci. 2024;19:436–46.
Asadi-Pooya AA, Malekpour M, Taherifard E, Mallahzadeh A, Kouhanjani MF. Coexistence of temporal lobe epilepsy and idiopathic generalized epilepsy. Epilepsy Behav. 2024;151:109602.
Malekpour M, Jafari A, Kashkooli M, Salarikia SR, Negahdaripour M. A systems biology approach for discovering the cellular and molecular aspects of psychogenic non-epileptic seizure. Front Psychiatry. 2023;14:1116892.
WHO. WHO. 2024. https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury.
Abroumand Gholami A, Tahmasebi F, Haghir H, Babaloo H. Targeting JAK/STAT signaling pathway by curcumin: Implications for spinal cord injury neuroprotection. Inflammopharmacology. 2025;33:1–19.
Jin Y, Song Y, Lin J, Liu T, Li G, Lai B, et al. Role of inflammation in neurological damage and regeneration following spinal cord injury and its therapeutic implications. Burn Trauma. 2023;11:tkac054.
Bhatt M, Sharma M, Das B. The role of inflammatory cascade and reactive astrogliosis in glial scar formation post-spinal cord injury. Cell Mol Neurobiol. 2024;44:1–18.
Shirzad S, Tayaranian Marvian M, Abroumand Gholami A, Gharehbaghi M, Marefati N, Salmani H, et al. Unveiling the effects of left hemispheric intracerebral hemorrhage on long-term potentiation and inflammation in the bilateral hippocampus: A preclinical study. J Stroke Cerebrovasc Dis [Internet]. 2024;33:107523. Available from: https://www.sciencedirect.com/science/article/pii/S105230572300544X.
Lukacova N, Kisucka A, Kiss Bimbova K, Bacova M, Ileninova M, Kuruc T, et al. Glial-neuronal interactions in pathogenesis and treatment of spinal cord injury. Int J Mol Sci. 2021;22:13577.
Xu S, Wang J, Jiang J, Song J, Zhu W, Zhang F, et al. TLR4 promotes microglial pyroptosis via lncRNA-F630028O10Rik by activating PI3K/AKT pathway after spinal cord injury. Cell Death Dis. 2020;11:693.
Abroumand Gholami A, Gheybi F, Molavi AM, Tahmasebi F, Papi A, Babaloo H. Effect of polycaprolactone/carbon nanotube scaffold implantation along with liposomal ellagic acid in hippocampal synaptogenesis after spinal cord injury. Nanomedicine J [Internet]. 2023;10:197–209. Available from: https://nmj.mums.ac.ir/article_22560.html.
Babaloo H, Barati S, Haghir H, Gholami AA, Moharreri P, Fallahnezhad S, et al. The effect of PU/MWCNT nanofiber scaffolds containing hesperidin nanoparticles and mesenchymal stem cells on the microglia and astrocyte phenotype in the spinal cord injury model. Neuroscience. 2025;583:53–62.
Wu W, Lee SY, Wu X, Tyler JY, Wang H, Ouyang Z, et al. Neuroprotective ferulic acid (FA)–glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35:2355–64.
Zou GJ, Chen ZR, Wang XQ, Cui YH, Li F, Li CQ, et al. Microglial activation in the medial prefrontal cortex after remote fear recall participates in the regulation of auditory fear extinction. Eur J Pharmacol [Internet]. 2024;978:176759. Available from: https://www.sciencedirect.com/science/article/pii/S0014299924004473.
Zeng WJ, Zhang L, Cao H, Li D, Zhang H, Xia Z, et al. A novel inflammation-related lncRNAs prognostic signature identifies LINC00346 in promoting proliferation, migration, and immune infiltration of glioma. Front Immunol. 2022;13:810572.
Mortezaee K, Khanlarkhani N, Beyer C, Zendedel A. Inflammasome: its role in traumatic brain and spinal cord injury. J Cell Physiol. 2018;233:5160–9.
Jiang W, Li M, He F, Zhou S, Zhu L. Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J Neuroinflammation. 2017;14:1–12.
Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase‐1 axis regulates IL‐1β processing in neutrophils. Eur J Immunol. 2012;42:710–5.
Luo J, Li X, Zhang L, Deng M, Zhao J, Zhang J, et al. 5-deoxy-rutaecarpine protects against LPS-induced acute lung injury via inhibiting NLRP3 inflammasome-related inflammation. Front Pharmacol. 2025;16:1522146.
Dominic A, Le NT, Takahashi M. Loop between NLRP3 inflammasome and reactive oxygen species. Antioxid Redox Signal. 2022;36:784–96.
Doyle TM, Chen Z, Durante M, Salvemini D. Activation of sphingosine-1-phosphate receptor 1 in the spinal cord produces mechanohypersensitivity through the activation of inflammasome and IL-1β pathway. J pain. 2019;20:956–64.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
Bascuñana P, Möhle L, Brackhan M, Pahnke J. Fingolimod as a treatment in neurologic disorders beyond multiple sclerosis. Drugs R D. 2020;20:197–207.
Malhotra S, Hurtado-Navarro L, Pappolla A, Villar LMM, Río J, Montalban X, et al. Increased NLRP3 inflammasome activation and pyroptosis in patients with multiple sclerosis with fingolimod treatment failure. Neurol Neuroimmunol Neuroinflammation. 2023;10:e200100.
Leo A, Citraro R, Marra R, Palma E, Donato Di Paola E, Constanti A, et al. The sphingosine 1-phosphate signaling pathway in epilepsy: A possible role for the immunomodulator drug fingolimod in epilepsy treatment. CNS Neurol Disord Targets-CNS Neurol Disord. 2017;16:311–25.
Doolen S, Iannitti T, Donahue RR, Shaw BC, Grachen CM, Taylor BK. Fingolimod reduces neuropathic pain behaviors in a mouse model of multiple sclerosis by a sphingosine-1 phosphate receptor 1-dependent inhibition of central sensitization in the dorsal horn. Pain. 2018;159:224–38.
Poormoghadam D, Shiadeh BR, Azedi F, Tavakol H, Rezayat SM, Tavakol S. Fingolimod nanoemulsions at different particle sizes define the fate of spinal cord injury recovery. Biomed Res Int. 2022;2022:5703426.
Li Y, Chen Y, Hu X, Ouyang F, Li J, Huang J, et al. Fingolimod (FTY720) hinders Interferon-γ-mediated fibrotic scar formation and facilitates neurological recovery after spinal cord injury. J Neurotrauma. 2023;40:2580–95.
Zeraatpisheh Z, Mirzaei E, Nami M, Alipour H, Mahdavipour M, Sarkoohi P, et al. Local delivery of fingolimod through PLGA nanoparticles and PuraMatrix‐embedded neural precursor cells promote motor function recovery and tissue repair in spinal cord injury. Eur J Neurosci. 2021;54:5620–37.
Gillespie ER, Ruitenberg MJ. Neuroinflammation after SCI: current insights and therapeutic potential of intravenous immunoglobulin. J Neurotrauma. 2022;39:320–32.
Efstathopoulos P, Kourgiantaki A, Karali K, Sidiropoulou K, Margioris AN, Gravanis A, et al. Fingolimod induces neurogenesis in adult mouse hippocampus and improves contextual fear memory. Transl Psychiatry. 2015;5:e685–e685.
Kong W, Qi Z, Xia P, Chang Y, Li H, Qu Y, et al. Local delivery of FTY720 and NSCs on electrospun PLGA scaffolds improves functional recovery after spinal cord injury. RSC Adv. 2019;9:17801–11.
Miranda RR, Ferreira NN, de Souza EE, Lins PMP, Ferreira LMB, Kruger A, et al. Modulating Fingolimod (FTY720) anti-SARS-CoV-2 activity using a PLGA-based drug delivery system. ACS Appl Bio Mater. 2022;5:3371–83.
Alipour H, Alizadeh A, Azarpira N, Saudi A, Alavi O, Tanideh N, et al. Incorporating fingolimod through poly (lactic‐co‐glycolic acid) nanoparticles in electrospun polyurethane/polycaprolactone/gelatin scaffold: An in vitro study for nerve tissue engineering. Polym Adv Technol. 2022;33:2589–600.
Sun J, Chen Q, Zhuang C, Li X, Yu L, Jin W. Mechanistic insights into synergistic effects using coupled PK-PD modeling. Sci Rep. 2025;15:15631.
He R, He F, Hu Z, He Y, Zeng X, Liu Y, et al. Analysis of potential mechanism of herbal formula Taohong Siwu decoction against vascular dementia based on network Pharmacology and molecular docking. Biomed Res Int. 2023;2023:1235552.
Shi Q, Hu T, Xu L, Fu J, Fang Y, Lan Y, et al. Fingolimod suppresses NLRP3 inflammasome activation and alleviates oxidative stress in traumatic brain injury-Induced acute lung injury. J Inflamm Res. 2025;18:2229–45.
Guo Y, Gan X, Zhou H, Zhou H, Pu S, Long X, et al. Fingolimod suppressed the chronic unpredictable mild stress-induced depressive-like behaviors via affecting microglial and NLRP3 inflammasome activation. Life Sci. 2020;263:118582.
Bahari Javan N, Rezaie Shirmard L, Jafary Omid N, Akbari Javar H, Rafiee Tehrani M, Abedin Dorkoosh F. Preparation, statistical optimisation and in vitro characterisation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/poly (lactic-co-glycolic acid) blend nanoparticles for prolonged delivery of teriparatide. J Microencapsul. 2016;33:460–74.
Khuyagbaatar B, Kim K, Kim YH. Conversion equation between the drop height in the New York University impactor and the impact force in the infinite horizon impactor in the contusion spinal cord injury model. J Neurotrauma. 2015;32:1987–93.
Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21.
Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci Off J Soc Neurosci. 2009;29:753–64.
Unal B, Kaplan S, Odaci E, Aslan H, Aksak S, Unal D, et al. Neuroprotective effects of methylprednisolone and hypothermia after experimental spinal cord injury: A histopathological and stereological study. Eurasian J Med. 2009;41:169–74.
Ramadan WS, Abdel-Hamid GA, Al-Karim S, Abbas AT. Histological, immunohistochemical and ultrastructural study of secondary compressed spinal cord injury in a rat model. Folia Histochem Cytobiol. 2017;55:11–20.
Ulndreaj A, Tzekou A, Mothe AJ, Siddiqui AM, Dragas R, Tator CH, et al. Characterization of the antibody response after cervical spinal cord injury. J Neurotrauma. 2017;34:1209–26.
Shi W, Sun Y, Wang J, Tang Y, Zhou S, Xu Z, et al. Trem1 mediates neuronal apoptosis via interaction with SYK after spinal cord ischemia-reperfusion injury. Am J Transl Res. 2021;13:6117.
Andrabi SS, Yang J, Gao Y, Kuang Y, Labhasetwar V. Nanoparticles with antioxidant enzymes protect injured spinal cord from neuronal cell apoptosis by attenuating mitochondrial dysfunction. J Control Release. 2020;317:300–11.
Zhang M, Bai Y, Xu C, Lin J, Jin J, Xu A, et al. Novel optimized drug delivery systems for enhancing spinal cord injury repair in rats. Drug Deliv. 2021;28:2548–61.
Moharreri P, Molavi AM, Abroumand Gholami A, Rahmani S, Mokhtari T, Gheybi F, et al. In vitro evaluation of bioactive PCL/alginate fibers with controlled liposomal silymarin release for mesenchymal stem cell transplantation. Sci Rep. 2025;15:35738.
Abroumand Gholami A, Molavi AM, Omrannezhad M, Mokhtari T, Rahmani S, Moharreri P, et al. Enhancing neural stem cell proliferation using Polyurethane/CNT scaffolds functionalized with liposomal hesperidin for neural tissue engineering. Nano Sel. 2025;7:e70103.
Rad AR, Mokhtari T, Amirazodi E, Molavi AM, Moghadam FM, Oskuee RK, et al. Hippocampal synaptic markers and cognitive recovery in spinal cord injury: the therapeutic potential of neural stem cell-laden carbon nanotube-based fiber scaffolds with liposomal hesperidin. Res Pharm Sci [Internet]. 2025;20. Available from: https://journals.lww.com/rips/fulltext/2025/09000/hippocampal_synaptic_markers_and_cognitive.10.aspx.
Hong Q, Song H, Chi NTL, Brindhadevi K. Numerous nanoparticles as drug delivery system to control secondary immune response and promote spinal cord injury regeneration. Process Biochem. 2022;112:145–53.
Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003.
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J Control release. 2016;235:34–47.
Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation. 2011;8:1–12.
Bravo GÁ, Cedeño RR, Casadevall MP, Ramió-Torrentà L. Sphingosine-1-phosphate (S1P) and S1P signaling pathway modulators, from current insights to future perspectives. Cells. 2022;11:2058.
Dong Y, Guo R, Ji J, Cao L, Zhang L, Chen Z, et al. S1 PR 3 is essential for phosphorylated fingolimod to protect astrocytes against oxygen‐glucose deprivation‐induced neuroinflammation via inhibiting TLR 2/4‐NF κB signalling. J Cell Mol Med. 2018;22:3159–66.
Sun L, Ma W, Gao W, Xing Y, Chen L, Xia Z, et al. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019;10:542.
Khatir AA, Abbasi A, Sarandili S, Sepidarkish M, Fazlollahpour-Naghibi A, Arjmandi D, et al. The association between Parkinson disease and Toxocara infection/exposure: A case-control study. J Helminthol. 2025;99:e40.
Moarrefzadeh A, Sarandili S, Motamed-Gorji N, Majdolashrafi F, Bahardoust M, Mousavi S, et al. Predictors of quality of life in patients with Parkinson’s disease: A multicenter case-control study. Basic Clin Neurosci. 2025;16:667.
Feng Y, Feng F, Pan S, Zhang J, Li W. Fingolimod ameliorates chronic experimental autoimmune neuritis by modulating inflammatory cytokines and Akt/mTOR/NF‐κB signaling. Brain Behav. 2023;13:e2965.
Luo H, Gu X, Tong G, Han L. Research progress of apelin in acute ischemic brain injury. Am J Transl Res. 2022;14:7260.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the overall conceptualization and study design. Mohammed Hussein M. Alsharbaty, Reem S. Alazragi, and Uday Abdul-Reda Hussein performed the experimental work, data acquisition, and initial data analysis. Siba Mekaael Yaseen assisted in histological assessment and interpretation of imaging data. Mustafa Mudhafar drafted the initial version of the manuscript and contributed to data visualization. Hasan Ali Alsailawi supervised the project, secured resources, and performed the critical revision of the manuscript for important intellectual content. All authors reviewed, edited, and approved the final manuscript and agree to be accountable for the integrity of the work.
Corresponding author
Ethics declarations
Ethics approval
All experimental procedures were approved by the Institutional Animal Ethics Committee of AlSafwa University College, Karbala, Iraq (Approval No. H15REA195).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
M. Alsharbaty, M.H., Alazragi, R.S., Mekaael yaseen, S. et al. Nanoformulated fingolimod attenuates NLRP3 inflammasome activation and promotes functional recovery in a rat model of spinal cord injury. Spinal Cord (2026). https://doi.org/10.1038/s41393-026-01177-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41393-026-01177-2