Abstract
Study design
Secondary analysis of aggregated case series data.
Objectives
To examine the effects of a high-fat/high-carbohydrate meal on leukocyte populations in adults with a chronic SCI.
Setting
University-based laboratories in British Columbia, Canada.
Methods
Ten individuals (M = 9) with a traumatic SCI (>1-year post-injury; M = 15.5 years; n = 2 sensory complete, n = 7 motor complete) participated in this study. Participants arrived fasted (≥12 h) prior to both the control (quiet sitting, no food/drink) and experimental meal conditions (high-fat/high-carb meal: 880 kcal, 52 g fat, 73 g carbohydrates, 29 g protein). Blood samples were taken in the fasted state and at 120-min post-meal/baseline in both conditions. Immune cell counts were assessed using multi-color flow cytometry.
Results
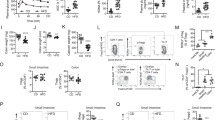
A significant time × condition interaction effect was seen in CD3+, CD4+, and CD8+ T cells as well as CD56+ and CD3+/CD56+ natural killer (NK) cells (p < 0.05). CD14+/CD16+ monocytes and CD19+ B cells approached a significant time × condition interaction (p < 0.07). A main effect of time was observed in CD19+ B cells (p < 0.05). Cell counts for T-lymphocytes and NK cells followed the general trend of an increase in the control condition from baseline to 120-min with no change observed following the experimental meal condition.
Conclusions
Following the HFHC meal, immune cells did not show the same general increase observed following the control condition. Future research is needed to determine if there are any potential consequences of these immune cell responses in immunosuppressed populations and if other factors (e.g., diurnal variation) might influence immune cell response.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
National Spinal Cord Injury Statistical Center. J Spinal Cord Med. 2002:139–40.
Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757–63. https://doi.org/10.1016/j.apmr.2004.03.016
Krueger H, Noonan VK, Trenaman LM, Joshi P, Rivers CS. The economic burden of traumatic spinal cord injury in Canada. Chronic Dis Inj Can. 2013;33:113–22.
Allison DJ, Ditor DS. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015;53:14–18. https://doi.org/10.1038/sc.2014.184
Cruse JM, Keith JC, Bryant ML, Lewis RE. Immune system-neuroendocrine dysregulation in spinal cord injury. Immunol Res. 1996;15:306–14.
Campagnolo DI, Bartlett JA, Keller SE. Influence of neurological level on immune function following spinal cord injury: a review. J Spinal Cord Med. 2000;23:121–8. https://doi.org/10.1080/10790268.2000.11753519
Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–64. https://doi.org/10.1016/J.BRAINRESREV.2005.11.004
Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res. 2014;58:136–46. https://doi.org/10.1002/mnfr.201300104
Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71:511–27. https://doi.org/10.1111/nure.12035
Allison DJ, Thomas A, Beaudry K, Ditor DS. Targeting inflammation as a treatment modality for neuropathic pain in spinal cord injury: a randomized clinical trial. J Neuroinflamm. 2016;13:152. https://doi.org/10.1186/s12974-016-0625-4
Martin Ginis KA, van der Scheer JW, Todd KR, Davis JC, Gaudet S, Hoekstra F, et al. A pragmatic randomized controlled trial testing the effects of the international scientific SCI exercise guidelines on SCI chronic pain: protocol for the EPIC-SCI trial. Spinal Cord. 2020;58:746–54. https://doi.org/10.1038/s41393-020-0478-7
Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56:308–21. https://doi.org/10.1038/s41393-017-0017-3
Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 2009;32:2281–7. https://doi.org/10.2337/dc09-0979
Walters JL, Buchholz AC, Martin Ginis KA. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord. 2009;47:318–22. https://doi.org/10.1038/sc.2008.134
Farkas GJ, Pitot MA, Berg AS, Gater DR. Nutritional status in chronic spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2019;57:3–17. https://doi.org/10.1038/s41393-018-0218-4
Fuller KNZ, Summers CM, Valentine RJ. Effect of a single bout of aerobic exercise on high-fat meal-induced inflammation. Metabolism. 2017;71:144–52. https://doi.org/10.1016/j.metabol.2017.03.001
Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4. https://doi.org/10.3389/fpsyg.2013.00863.
Oostrom AJHHMvan, Rabelink TJ, Verseyden C, Sijmonsma TP, Plokker HWM, De Jaegere PPTh, et al. Activation of leukocytes by postprandial lipemia in healthy volunteers. Atherosclerosis. 2004;177:175–82. https://doi.org/10.1016/j.atherosclerosis.2004.07.004
Hansen K, Sickelmann F, Pietrowsky R, Fehm HL, Born J. Systemic immune changes following meal intake in humans. Am J Physiol. 1997;273:R548–53. https://doi.org/10.1152/ajpregu.1997.273.2.R548
Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92. https://doi.org/10.1093/ajcn/86.5.1286
Sennels HP, Jørgensen HL, Hansen A-LS, Goetze JP, Fahrenkrug J. Diurnal variation of hematology parameters in healthy young males: the Bispebjerg study of diurnal variations. Scand J Clin Lab Investig. 2011;71:532–41. https://doi.org/10.3109/00365513.2011.602422
Plumelle D, Lombard E, Nicolay A, Portugal H. Influence of diet and sample collection time on 77 laboratory tests on healthy adults. Clin Biochem. 2014;47:31–7. https://doi.org/10.1016/j.clinbiochem.2013.11.002
Fatima G, Sharma VP, Verma NS. Circadian variations in melatonin and cortisol in patients with cervical spinal cord injury. Spinal Cord. 2016;54:364–7. https://doi.org/10.1038/sc.2015.176
Kostovski E, Frigato E, Savikj M, Dahm AEA, Sandset PM, Mowinckel M-C, et al. Normalization of disrupted clock gene expression in males with tetraplegia: a crossover randomized placebo-controlled trial of melatonin supplementation. 2018. https://doi.org/10.1038/s41393-018-0176-x.
Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS. Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med. 2000;23:129–35. https://doi.org/10.1080/10790268.2000.11753520
Koszinowski UH, Reddehase MJ, Jonjic S. The role of CD4 and CD8 T cells in viral infections. Curr Opin Immunol. 1991;3:471–5. https://doi.org/10.1016/0952-7915(91)90005-L
Bancroft GJ. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–10. https://doi.org/10.1016/0952-7915(93)90030-V
Berg-Emons RJvanden, Bussmann JB, Stam HJ. Accelerometry-based activity spectrum in persons with chronic physical conditions. Arch Phys Med Rehabil. 2010;91:1856–61. https://doi.org/10.1016/j.apmr.2010.08.018
Nash MS, Groah SL, Gater DR, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and management of cardiometabolic risk after spinal cord injury: clinical practice guideline for health care providers. Top Spinal Cord Inj Rehabil. 2018;24:379–423. https://doi.org/10.1310/sci2404-379
Chan W-M, Mohammed Y, Lee I, Pearse DD. Effect of gender on recovery after spinal cord injury. Transl Stroke Res. 2013;4:447–61. https://doi.org/10.1007/s12975-012-0249-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dix, G.U., Jackson, G.S., Todd, K.R. et al. The effects of a high-fat/high-carbohydrate meal on leukocyte populations in adults with chronic spinal cord injury. Spinal Cord Ser Cases 7, 49 (2021). https://doi.org/10.1038/s41394-021-00412-7
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41394-021-00412-7