Abstract
Study design
Retrospective study.
Objectives
We aimed to characterize the convergent disruptions of the structural connectivity based on network modeling technique (i.e., graph theory) to identify significant changes in network organization/reorganization between uninjured and chronic spinal cord injury (SCI) participants.
Setting
USA.
Methods
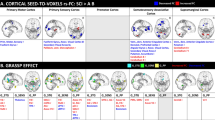
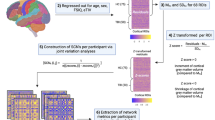
Ten adult participants including 4 with chronic SCI and 6 uninjured were scanned using a multi-shell diffusion imaging on a 3.0 T MR scanner. Whole brain structural connectivity matrix was estimated by performing the quantification of the number of white matter fibers (called edges) connecting each possible pair of brain region (called nodes). Brain regions were defined according to Desikan–Killiany cortical atlas. Using connectivity matrix, connectivity strength as well as six different graph theoretical measurements were computed for each participant. They include: (1) global efficiency; (2) local efficiency; (3) degree; (4) betweenness centrality; (5) average shortest length and (6) clustering coefficient. Finally network based statistics was applied to extract nodes/connections with significant differences between groups (uninjured vs SCI).
Results
The SCI group showed significant decreases in betweenness centrality in the left precentral gyrus (T-score=2.98, p value=0.02), and the right caudal middle frontal gyrus (score = 2.35, p value=0.047). It also showed significant decrease in left transverse temporal gyrus (T-score=2.36, p value=0.046) in clustering coefficient. In addition, altered regions in the occipital and parietal lobe were also identified.
Conclusion
These results suggest that not only local but also global alterations of the white matter occur after SCI. The proposed modeling technique has the potential to serve as a screening tool to identify any areas of the brain affected after SCI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nardone R, Höller Y, Sebastianelli L, Versace V, Saltuari L, Brigo F, et al. Cortical morphometric changes after spinal cord injury. Brain Res Bull. 2018;137:107–19.
Nardone R, Höller Y, Brigo F, Seidl M, Christova M, Bergmann J, et al. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res. 2013;1504:58–73.
Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, et al. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–9.
Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury: an electroencephalographic study. Neurology. 1998;50:1115–21.
Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, et al. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–32.
Kokotilo KJ, Eng JJ, Curt A. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma. 2009;26:2113–26.
Athanasiou A, Klados MA, Pandria N, Foroglou N, Kavazidi KR, Polyzoidis K, et al. A systematic review of investigations into functional brain connectivity following spinal cord injury. Front Hum Neurosci. 2017;11:1–9.
Hawasli AH, Rutlin J, Roland JL, Murphy RKJ, Song SK, Leuthardt EC, et al. Spinal cord injury disrupts resting-state networks in the human brain. J Neurotrauma. 2018;35:864–73.
He Y, Evans A. Graph theoretical modeling of brain connectivity. Curr Opin Neurol. 2010;23:341–50.
Chiang S, Haneef Z. Graph theory findings in the pathophysiology of temporal lobe epilepsy. Clin Neurophysiol. 2014;125:1295–305.
Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci. 2019;13:1–27.
Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98.
Kaushal M, Oni-Orisan A, Chen G, Li W, Leschke J, Ward D, et al. Large-scale network analysis of whole-brain resting-state functional connectivity in spinal cord injury: a comparative study. Brain Connect. 2017;7:413–23.
Kaushal M, Oni-Orisan A, Chen G, Li W, Leschke J, Ward BD, et al. Evaluation of whole-brain resting-state functional connectivity in spinal cord injury: a large-scale network analysis using network-based statistic. J Neurotrauma. 2017b;34:1278–82.
Kamagata K, Zalesky A, Hatano T, Di Biase MA, El Samad O, Saiki S, et al. Connectome analysis with diffusion MRI in idiopathic Parkinson’s disease: evaluation using multi-shell, multi-tissue, constrained spherical deconvolution. Neuroimage Clin. 2017;17:518–29.
Sinke MRT, Otte WM, Christiaens D, Schmitt O, Leemans A, van der Toorn A, et al. Diffusion MRI-based cortical connectome reconstruction: dependency on tractography procedures and neuroanatomical characteristics. Brain Struct Funct. 2018;223:2269–85.
Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:1–16.
Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9.
Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–73.
Perani D, Brunelli GA, Tettamanti M, Scifo P, Tecchio F, Rossini PM, et al. Remodelling of sensorimotor maps in paraplegia: a functional magnetic resonance imaging study after a surgical nerve transfer. Neurosci Lett. 2001;303:62–6.
Ding Y, Kastin AJ, Pan W. Neural plasticity after spinal cord injury. Curr Pharm Des. 2005;11:1441–50.
Craig A, Guest R, Tran Y, Middleton J. Cognitive impairment and mood states after spinal cord injury. J Neurotrauma. 2017;34:1156–63.
Nicotra A, Critchley HD, Mathias CJ, Dolan RJ. Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain. 2006;129:718–28.
Nakata H, Sakamoto K, Kakigi R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front Psychol. 2014;5:1–12.
Chiaravalloti ND, Weber E, Wylie G, Dyson-Hudson T, Wecht JM. Patterns of cognitive deficits in persons with spinal cord injury as compared with both age-matched and older individuals without spinal cord injury. J Spinal Cord Med. 2020;43:88–97.
Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32.
Wecht JM, Bauman WA. Decentralized cardiovascular autonomic control and cognitive deficits in persons with spinal cord injury. J Spinal Cord Med. 2013;36:74–81.
Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. J Int Neuropsychol Soc. 1997;3:464–72.
Bartolo R, Saunders RC, Mitz AR, Averbeck BB. Dimensionality, information and learning in prefrontal cortex. PLoS Comput Biol. 2020;16:1–26.
Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–69.
Min YS, Chang Y, Park JW, Lee JM, Cha J, Yang JJ, et al. Change of brain functional connectivity in patients with spinal cord injury: graph theory based approach. Ann Rehabil Med. 2015;39:374–83.
Richards JS, Seitz MR, Eisele WA. Auditory processing in spinal cord injury: a preliminary investigation from a sensory deprivation perspective. Arch Phys Med Rehabil. 1986;67:115–7.
De Vico Fallani F, Astolfi L, Cincotti F, Mattia D, Marciani MG, Salinari S, et al. Cortical functional connectivity networks in normal and spinal cord injured patients: evaluation by graph analysis. Hum Brain Mapp. 2007;28:1334–46.
Funding
This work was supported by provost pilot clinical research grant, Thomas Jefferson University. We also acknowledge support by NIH NINDS #NS097880 (MRD) and Department of Defense, Spinal Cord Injury Research Program Award No. W81XWH-17-1-0476.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh, M., Manmatharayan, A.R., Johnston, T. et al. Graph theoretical structural connectome analysis of the brain in patients with chronic spinal cord injury: preliminary investigation. Spinal Cord Ser Cases 7, 60 (2021). https://doi.org/10.1038/s41394-021-00424-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41394-021-00424-3