Abstract
Study design
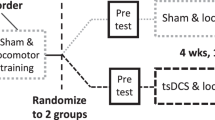
A randomized, sham-controlled clinical trial.
Objective
To test the effects of tDCS, combined with robotic training, on gait disability in SCI. Our hypothesis was that participants who received active tDCS would experience greater walking gains, as indexed by the WISCI-II, than those who received sham tDCS.
Setting
University of São Paulo, Brazil.
Methods
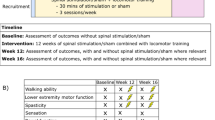
This randomized, double-blind study comprised 43 participants with incomplete SCI who underwent 30 sessions of active (n = 21) or sham (n = 22) tDCS (20 min, 2 mA) before every Lokomat session of 30 min (3 times a week over 12 weeks or 5 times a week over 6 weeks). The main outcome was the improvement in WISCI-II. Participants were assessed at baseline, after 15 and 30 sessions of Lokomat, and after three months of treatment.
Results
There was a significant difference in the percentage of participants that improved in WISCI-II at the 30-session, compared with baseline: 33.3% in the sham group and 70.0% in the active group (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). At the follow-up, the improvement compared with baseline in the sham group was 35.0% vs. 68.4% for the active group (p = 0.046; OR: 3.7; 95% CI: 1.0–13.5). There was no significant difference at the 15-session.
Conclusion
Thirty sessions of active tDCS is associated with a significant improvement in walking, compared to sham. Moreover, 15 sessions had no significant effect. The improvement in WISCI-II can be related to different aspects of motor learning, including motor recovery and compensation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–31.
Hesse S, Werner C. Connecting research to the needs of patients and clinicians. Brain Res Bull. 2009;78:26–34.
Nam KY, Kim HJ, Kwon BS, Park JW, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14:24.
Leite VF, Souza DR, Imamura M, Battistella LR. Post-discharge mortality in patients with traumatic spinal cord injury in a Brazilian hospital: a retrospective cohort. Spinal Cord. 2019;57:134–140. https://pubmed.ncbi.nlm.nih.gov/30089892/.
Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology.2001;57:1899–901.
Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–4.
Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, et al. Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol. 2017;128:2318–29.
Martin JH. Harnessing neural activity to promote repair of the damaged corticospinal system after spinal cord injury. Neural Regen Res. 2016;11:1389–91.
Yozbatiran N, Keser Z, Davis M, Stampas A, O’Malley MK, Cooper-Hay C, et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: a proof of concept sham-randomized clinical study. NeuroRehabilitation.2016;39:401–11.
Barron HC, Vogels TP, Emir UE, Makin TR, O’Shea J, Clare S, et al. Unmasking latent inhibitory connections in human cortex to reveal dormant cortical memories. Neuron.2016;90:191–203.
Ammann C, Spampinato D, Márquez-Ruiz J. Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front Psychol. 2016;7:1981.
Kumru H, Murillo N, Benito-Penalva J, Tormos JM, Vidal J. Transcranial direct current stimulation is not effective in the motor strength and gait recovery following motor incomplete spinal cord injury during Lokomat(®) gait training. Neurosci Lett. 2016;620:143–7.
Raithatha R, Carrico C, Powell ES, Westgate PM, Chelette Ii KC, Lee K, et al. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study. NeuroRehabilitation.2016;38:15–25.
Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation (tDCS) in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24:256–313. https://pubmed.ncbi.nlm.nih.gov/32710772/.
Simis M T107. Using Functional near Infrared Spectroscopy (fNIRS) to assess brain activity of spinal cord injury patient, during robot-assisted gait. In: Santos K, Sato J, Fregni F, Battistella L, editors. Clinical Neurophysiology: Elsevier; 2018. p. e43–e4.
Simis M, Uygur-Kucukseymen E, Pacheco-Barrios K, Battistella LR, Fregni F. Beta-band oscillations as a biomarker of gait recovery in spinal cord injury patients: a quantitative electroencephalography analysis. Clin Neurophysiol. 2020;131:1806–14.
Burns AS, Delparte JJ, Patrick M, Marino RJ, Ditunno JF. The reproducibility and convergent validity of the walking index for spinal cord injury (WISCI) in chronic spinal cord injury. Neurorehabil Neural Repair. 2011;25:149–57.
Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ.2012;345:e5840.
Gomes-Osman J, Field-Fote EC. Cortical vs. afferent stimulation as an adjunct to functional task practice training: a randomized, comparative pilot study in people with cervical spinal cord injury. Clin Rehabil. 2015;29:771–82.
Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141.
Bai S, Dokos S, Ho KA, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. Neuroimage.2014;87:332–44.
Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-element model predicts current density distribution for clinical applications of tDCS and tACS. Front Psychiatry. 2012;3:83.
Castillo-Saavedra L, Gebodh N, Bikson M, Diaz-Cruz C, Brandao R, Coutinho L, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: phase II open-label dose optimization. J Pain. 2016;17:14–26.
Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham-controlled longitudinal clinical trial. J Pain Manag. 2009;2:353–61.
de Paz RH, Serrano-Muñoz D, Pérez-Nombela S, Bravo-Esteban E, Avendaño-Coy J, Gómez-Soriano J. Combining transcranial direct-current stimulation with gait training in patients with neurological disorders: a systematic review. J Neuroeng Rehabil. 2019;16:114.
Agboada D, Mosayebi Samani M, Jamil A, Kuo MF, Nitsche MA. Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci Rep. 2019;9:18185.
Shmuelof L, Krakauer JW. Are we ready for a natural history of motor learning? Neuron.2011;72:469–76.
Spampinato D, Celnik P. Multiple motor learning processes in humans: defining their neurophysiological bases. Neuroscientist. 2020. https://doi.org/10.1177/1073858420939552.
Dhawale AK, Smith MA, Ölveczky BP. The role of variability in motor learning. Annu Rev Neurosci. 2017;40:479–98.
Kumru H, Benito-Penalva J, Valls-Sole J, Murillo N, Tormos JM, Flores C, et al. Placebo-controlled study of rTMS combined with Lokomat. Exp Brain Res. 2016;234:3447–55.
Benito J, Kumru H, Murillo N, Costa U, Medina J, Tormos JM, et al. Motor and gait improvement in patients with incomplete spinal cord injury induced by high-frequency repetitive transcranial magnetic stimulation. Top Spinal Cord Inj Rehabil. 2012;18:106–12.
Korzhova J, Sinitsyn D, Chervyakov A, Poydasheva A, Zakharova M, Suponeva N, et al. Transcranial and spinal cord magnetic stimulation in treatment of spasticity: a literature review and meta-analysis. Eur J Phys Rehabil Med. 2018;54:75–84.
Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair. 2010;24:435–41.
Nardone R, Höller Y, Thomschewski A, Brigo F, Orioli A, Höller P, et al. rTMS modulates reciprocal inhibition in patients with traumatic spinal cord injury. Spinal Cord. 2014;52:831–5.
Acknowledgements
Authors are grateful to Margarida H. Miyazaki, executive director of IMREA and Katia Lina Miyahara, Clinical Director of IMREA, for the great support of the hospital. Besides, Artur dos Santos, Antonio Migliorati, Daniel de Souza, Denise Matheus, Gerson Barbieri Filho, Karin Santos, Mariana Milazzotto, Patricia Monteiro Marchiore, Thais Terranova, Tharsila de Oliveira and Vivian da Silva for assistance with participant data collection and study monitoring.
Funding
This work was supported by grants from Ministry of Health (SIPAR: 25000.160.761/2014-54). The researchers received support from the São Paulo Research Foundation (FAPESP- SPEC, grant #2017/12943-8). The work received a support from Núcleo de Apoio a Pesquisa-Núcleo de Estudos Avançados em Reabilitação (NAP-NEAR).
Author information
Authors and Affiliations
Contributions
MS, FF, and LB conceptualized the paper. MS and LB applied the therapies. MS and FF analyzed the data. LCB and FF provided a critical review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. This study was approved by the Ethics Committee for Analysis of Research Projects of the University of São Paulo Medical School.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Simis, M., Fregni, F. & Battistella, L.R. Transcranial direct current stimulation combined with robotic training in incomplete spinal cord injury: a randomized, sham-controlled clinical trial. Spinal Cord Ser Cases 7, 87 (2021). https://doi.org/10.1038/s41394-021-00448-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00448-9
This article is cited by
-
Global trends and hotspots of neuromodulation in spinal cord injury: a study based on bibliometric analysis
Journal of Orthopaedic Surgery and Research (2025)
-
Non-invasive cerebral and spinal cord stimulation for motor and gait recovery in incomplete spinal cord injury: systematic review and meta-analysis
Journal of NeuroEngineering and Rehabilitation (2025)
-
Effects of non-invasive brain stimulation on motor function after spinal cord injury: a systematic review and meta-analysis
Journal of NeuroEngineering and Rehabilitation (2023)