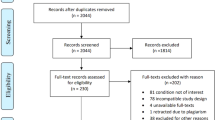
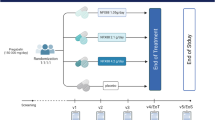
Abstract
Study Design
Observational, longitudinal, prospective study.
Objectives
This study aimed to evaluate the effects of Nabiximols (Sativex®) on spasticity in chronic spinal cord injury (SCI) individuals refractory to conventional therapy. Secondary objectives included assessing its impact on functional independence, neuropathic pain, sleep quality, and depression.
Setting
Institute Guttmann, a neurorehabilitation hospital in Badalona, Catalonia (Spain).
Methods
Adult participants ( >18 years) with chronic SCI ( >6 months) and moderate to severe spasticity refractory to conventional treatments were recruited. All participants underwent baseline assessments and were followed up at one and two months after initiating treatment with nabiximols oromucosal spray, with individualised dose adjustments on a weekly basis. Assessed variables included spasticity, functional independence, neuropathic pain, sleep quality, depression, quality of life, and Patient Global Impression of Improvement (PGI-I).
Results
Statistically significant improvements in spasticity were observed after one month (VAS decrease of 30%, p < 0.001; MAS decrease of 60%, p = 0.001) and two months (VAS decrease of 30%, p < 0.001; MAS decrease of 52%, p = 0.011) of treatment. A positive PGI-I was reported in 67% of participants. However, no significant changes were noted in spasms frequency, functional independence, neuropathic pain, or sleep quality. No significant differences in spasticity change or non-motor symptoms were found between participants with complete and incomplete SCI.
Conclusions
Nabiximols may effectively reduce spasticity in individuals with SCI resistant to conventional therapies. Given the significant impact of spasticity associated with SCI, it could be considered a viable add-on therapy for this population.
Trial registry
This clinical trial is registered in the EudraCT database under the identifier 2022-001777-31.
Sponsorship
Almirall, S.A., Barcelona, Catalonia (Spain).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Schnelle M, Grotenhermen F, Reif M, Gorter RW. Ergebnisse einer standardisierten Umfrage zur medizinischen Verwendung von Cannabisprodukten im deutschen Sprachraum. Complement Med Res. 1999;6:28–36.
Grotenhermen F, Reckendrees B Die Behandlung mit Cannabis und THC: medizinische Möglichkeiten, rechtliche Lage, Rezepte, Praxistipps. 6., erweiterte Neuauflage. Solothurn: Nachtschatten Verlag; 2016. 105 p.
Chiurchiù V, Van Der Stelt M, Centonze D, Maccarrone M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Progress Neurobiol. 2018;160:82–100.
Zou S, Kumar U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. International J Mol Sci. 2018;19:833.
Friedman D, French JA, Maccarrone M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019;18:504–12.
Mecha M, Carrillo-Salinas FJ, Feliú A, Mestre L, Guaza C. Perspectives on Cannabis-Based Therapy of Multiple Sclerosis: A Mini-Review. Front Cell Neurosci. 2020;14:34.
Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999–1012.
Grotenhermen F, Müller-Vahl K The Therapeutic Potential of Cannabis and Cannabinoids. Deutsches Ärzteblatt international [Internet]. 2012 Jul 23 [cited 2024 May 27]; Available from: https://www.aerzteblatt.de/10.3238/arztebl.2012.0495.
Karst M, Bernateck M. Schmerzlinderung durch Cannabinoide? Bedeutung des Endocannabinoid systems und der Cannabinoide für die Schmerztherapie. Anästhesiol Intensivmed Notfallmed Schmerzther. 2008;43:522–8.
Harris CR, Brown A. Synthetic Cannabinoid Intoxication: A Case Series and Review. Journal Emerg Med. 2013;44:360–6.
Robson P. Abuse potential and psychoactive effects of δ-9-tetrahydrocannabinol and cannabidiol oromucosal spray (Sativex), a new cannabinoid medicine. Expert Opin Drug Saf. 2011;10:675–85.
HERRERO R. Sanidad aprueba un derivado del cannabis para tratar la esclerosis múltiple [Internet]. El Correo. 2010 [cited 2024 May 27]. Available from: https://www.elcorreo.com/vizcaya/v/20100729/sociedad/sanidad-aprueba-derivado-cannabis-20100729.html.
Langley GB, Sheppeard H. The visual analogue scale: Its use in pain measurement. Rheumatol Int. 1985;5:145–8.
Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Research Nurs Health. 1990;13:227–36.
Harb A, Kishner S Modified Ashworth Scale. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Sep 3]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK554572/.
Mills PB, Vakil AP, Phillips C, Kei L, Kwon BK. Intra-rater and inter-rater reliability of the Penn Spasm Frequency Scale in People with chronic traumatic spinal cord injury. Spinal Cord. 2018;56:569–74.
Khamnon N, Amatachaya S, Wattanapan P, Musika N, Jitmongkolsri P, Kongngoen N, et al. Reliability and concurrent validity of the Spinal Cord Independence Measure III among rehabilitation professionals. Spinal Cord. 2022;60:875–81.
Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–57.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13.
The Whoqol Group. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol Med. 1998;28:551–8.
Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Nuerosci. 1990;240:1–4.
Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–9.
Patti F, Chisari CG, Solaro C, Benedetti MD, Berra E, Bianco A, et al. Effects of THC/CBD oromucosal spray onspasticity-related symptoms in people with multiple sclerosis: results from a retrospective multicenter study. Neurol Sci. 2020;41:2905–13.
Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord. 2007;45:551–62.
Pooyania S, Ethans K, Szturm T, Casey A, Perry D. A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury. Arch Phys Med Rehabil. 2010;91:703–7.
Abood ME, Pertwee RG, editors. Cannabinoids. Berlin Heidelberg: Springer; 2005. 770 p. (Handbook of experimental pharmacology / ed. board G. V. R. Born).
Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An Exploratory Human Laboratory Experiment Evaluating Vaporized Cannabis in the Treatment of Neuropathic Pain From Spinal Cord Injury and Disease. J Pain. 2016;17:982–1000.
Rintala DH, Fiess RN, Tan G, Holmes SA, Bruel BM. Effect of Dronabinol on Central Neuropathic Pain After Spinal Cord Injury: A Pilot Study. American J Phys Med Rehabilitation. 2010;89:840–8.
Hansen JS, Gustavsen S, Roshanisefat H, Kant M, Biering-Sørensen F, Andersen C, et al. Cannabis-Based Medicine for Neuropathic Pain and Spasticity—A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Trial. Pharmaceuticals. 2023;16:1079.
Bourke JA, Catherwood VJ, Nunnerley JL, Martin RA, Levack WMM, Thompson BL, et al. Using cannabis for pain management after spinal cord injury: a qualitative study. Spinal Cord Ser Cases. 2019;5:82.
Thomas PA, Carter GT, Bombardier CH. A scoping review on the effect of cannabis on pain intensity in people with spinal cord injury. Journal Spinal Cord Med. 2022;45:656–67.
Novotna A, Mares J, Ratcliffe S, Novakova I, Vachova M, Zapletalova O. et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols *(Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122–31.
Mallada Frechín J. Effect of Tetrahydrocannabinol:Cannabidiol Oromucosal Spray on Activities of Daily Living in Multiple Sclerosis Patients with Resistant Spasticity: A retrospective, Observational Study. Neurodegener Dis Manag. 2018;8:151–9.
Villan S. Use of Δ9-tetrahydrocannabinol in the treatment of spasticity in spinal cord injury patients. Spinal Cord. 2008;46:460–460.
Acknowledgements
We express our gratitude to Dr Gabriele Cattaneo (Institut Guttmann Barcelona) for his valuable assistance in statistical management.
Funding
The medication used for this study was provided by Almirall, S.A., Barcelona, Catalonia (Spain).
Author information
Authors and Affiliations
Contributions
Listed authors have met the following authorship criteria: (1) JVS, JBP and SA conceived and designed the work that led to the submission, (2) FMB acquired data, (3) SA, JVS and FMB played an important role in interpreting the results, (4) FMB drafted the paper, (5) JVS, SA, CMF and VMS revised the paper and approved the final version, (6) JVS and FMB agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Ethics approval was obtained from the Ethics Committee for Research with Medicinal Products (CEIm) of the Fundació Unió Catalana d’Hospitals, by resolution dated 18 April 2023. This study is registered in the EudraCT database under number 2022-001777-31. Written informed consent was obtained from all participants prior to participation. No identifiable images or personal data of participants are included in this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martins Braga, F., Albu, S., Mariño Fernández, C. et al. The effects of nabiximols (Sativex®) on spasticity and non-motor symptoms in chronic spinal cord injury (SCI): a longitudinal prospective study. Spinal Cord Ser Cases 11, 19 (2025). https://doi.org/10.1038/s41394-025-00712-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41394-025-00712-2