Abstract
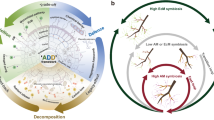
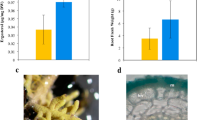
Symbiotic ectomycorrhizal fungi have received increasing attention as regulators of below-ground organic matter storage. They are proposed to promote organic matter accumulation by suppressing saprotrophs, but have also been suggested to play an active role in decomposition themselves. Here we show that exclusion of tree roots and associated ectomycorrhizal fungi in a boreal forest increased decomposition of surface litter by 11% by alleviating nitrogen limitation of saprotrophs–a “Gadgil effect”. At the same time, root exclusion decreased Mn-peroxidase activity in the deeper mor layer by 91%. Our results show that ectomycorrhizal fungi may hamper short-term litter decomposition, but also support a crucial role of ectomycorrhizal fungi in driving long-term organic matter oxidation. These observations stress the importance of ectomycorrhizal fungi in regulation of below-ground organic matter accumulation. By different mechanisms they may either hamper or stimulate decomposition, depending upon stage of decomposition and location in the soil profile.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Averill C, Turner BL, Finzi AC. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature. 2014;505:543–5.
Lin G, McCormack ML, Ma C, Guo D. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 2016;213:1440–51.
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model‐based assessment. Ecol Lett. 2011;14:493–502.
Gadgil RL, Gadgil PD. Mycorrhiza and litter decomposition. Nature. 1971;233:133.
Averill C, Hawkes CV. Ectomycorrhizal fungi slow soil carbon cycling. Ecol Lett. 2016;19:937–47.
Kyaschenko J, Clemmensen KE, Karltun E, Lindahl BD. Guild interaction within fungal communities regulates below-ground organic matter accumulation along a boreal forest fertility gradient. Ecol Lett. 2017b. https://doi.org/10.1111/ele.12862.
Hartley IP, Garnett MH, Sommerkorn M, Hopkins DW, Fletcher BJ, Sloan VL, et al. A potential loss of carbon associated with greater plant growth in the European Arctic. Nature. Clim Change. 2012;2:875–9.
Subke JA, Voke NR, Leronni V, Garnett MH, Ineson P. Dynamics and pathways of autotrophic and heterotrophic soil CO2 efflux revealed by forest girdling. J Ecol. 2011;99:186–93.
Brzostek ER, Dragoni D, Brown ZA, Phillips RP. Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol. 2015;206:1274–82.
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015;205:1525–36.
Parker T, Subke JA, Wookey PA. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob Change Biol. 2015;21:2070–81.
Fernandez CW, Kennedy PG. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol. 2016;209:1382–94.
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, et al. A large and persistent carbon sink in the World’s forests. Science. 2011;333:988–93.
Clemmensen KE, Bahr A, Ovaskainen O, Dahlberg A, Ekblad A, Wallander H, et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science. 2013;339:1615–8.
Wardle DA, Hörnberg G, Zackrisson O, Kalela-Brundin M, Coomes DA. Long-term effects of wildfire on ecosystem properties across an island area gradient. Science. 2003;300:972–5.
Fierer N, Strickland MS, Liptzin D, Bradford MA, Cleveland CC. Global patterns in belowground communities. Ecol Lett. 2009;12:1238–49.
Sterkenburg E, Bahr A, Brandström–Durling M, Clemmensen KE, Lindahl BD. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015;207:1145–58.
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, et al. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336:1715–9.
Cooke RC, Rayner ADM. Ecology of saprotrophic fungi. London: Longman; 1984.
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Finlay RD. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 2007;173:611–20.
Bödeker ITM, Lindahl BD, Olson Å, Clemmensen KE. Mycorrhizal and saprotrophic fungal guilds compete for the same organic substrates but affect decomposition differently. Funct Ecol. 2016;30:1967–78.
Leake JR, Donnelly DP, Saunders EM, Boddy L, Read DJ. Rates and quantities of carbon flux to ectomycorrhizal mycelium following 14C pulse labeling of Pinus sylvestris seedlings: effects of litter patches and interaction with a wood-decomposer fungus. Tree Physiol. 2001;21:71–82.
Lindahl B, Stenlid J, Finlay R. Effects of resource availability on mycelial interactions and 32P-transfer between a saprotrophic and an ectomycorrhizal fungus in soil microcosms. FEMS Microbiol Ecol. 2001;38:43–52.
Boberg JB, Finlay RD, Stenlid J, Ekblad A, Lindahl BD. Nitrogen and carbon reallocation in fungal mycelia during decomposition of boreal forest litter. PLoS One. 2014;9:e92897.
Bhupinderpal-Singh NordgrenA, Ottosson Löfvenius M, Högberg MM, Mellander P-E, Högberg P. Tree root and soil heterotrophic respiration as revealed by girdling of boreal Scots pine forest: extending observations beyond the first year. Plant Cell Environ. 2003;26:1287–96.
Kyaschenko J, Clemmensen KE, Hagenbo A, Karltun E, Lindahl BD. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017a;11:863–74.
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet. 2015;47:410–5.
Martin F, Kohler S, Murat C, Veneault-Fourrey C, Hibbett DS. Unearthing the roots of ectomycorrhizal symbioses. Nat Rev Microbiol. 2016;14:760–73.
Romell LG. A trenching experiment in a spruce forest and its bearing on problems of mycotrophy. Sven Bot Tidskr. 1938;32:89–99.
Clemmensen KE, Ihrmark K, Brandström-Durling M, Lindahl BD. Sample preparation for fungal community analysis by high-throughput sequencing of barcode amplicons. In: Martin F, Uroz S, editors. Microbial Environmental Genomics (MEG). New York: Springer; 2016. p. 61–88.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols. A Guide to Methods and Applications. Cambridge: Elsevier; 1990. p. 315–22.
Ihrmark K, Bödeker IT, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, et al. New primers to amplify the fungal ITS2 region – evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol. 2012;82:666–77.
Frank DN. BARCRAWL and BARTAB: software for design and implementation of barcoded primers for highly multiplexed DNA sequencing. BMC Bioinforma. 2009;10:362.
Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1.
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2013;22:5271–7.
Abarenkov K, Tedersoo L, Nilsson RH, Vellak K, Saar I, Veldre V, et al. PlutoF-a web based workbench for ecological and taxonomic research, with an online implementation for fungal ITS sequences. Evol Bioinform. 2010;6:189–96.
Nylund JE, Wallander H. Ergosterol analysis as a means of quantifying mycorrhizal biomass. Method Microbiol. 1992;24:77–88.
Saiya-Cork KR, Sinsabaugh RL, Zak DR. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem. 2002;34:1309–15.
Lindahl BD, de Boer W, Finlay RD. Disruption of root carbon transport into forest humus stimulates fungal opportunists at the expense of mycorrhizal fungi. ISME J. 2010;4:872–81.
Högberg P, Högbom L, Schinkel H, Högberg M, Johannisson C, Wallmark H. 15N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia. 1996;108:207–14.
Hobbie EA, Colpaert JV. Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol. 2003;157:115–26.
Näsholm T, Högberg P, Franklin O, Metcalfe D, Keel SG, Campbell C, et al. Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests? New Phytol. 2013;198:214–21.
Baldrian P, Kolařík M, Stursová M, Kopecký J, Valášková V, Větrovský T, et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012;6:248–58.
Stendahl J, Berg B, Lindahl BD. Manganese availability is negatively associated with carbon storage in northern coniferous forest humus layers. Sci Rep. 2017;7:15487.
Pellitier PT, Zak DR. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytol. 2017; https://doi.org/10.1111/nph.14598.
Frank B. Die Bedeutung der Mykorhiza Pilze für die gemeine Kiefer. Forstwiss Cent. 1894;16:185–90.
Shah F, Nicolás C, Bentzer J, Ellström M, Smits M, Rineau F, et al. Ectomycorrhizal fungi decompose soil organic matter using oxidative mechanisms adapted from saprotrophic ancestors. New Phytol. 2016;209:1705–19.
Bödeker ITM, Nygren CMR, Taylor AFS, Olson Å, Lindahl BD. ClassII peroxidase encoding genes are present in a wide phylogenetic range of ectomycorrhizal fungi. ISME J. 2009;3:1387–95.
Sinsabaugh RL. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem. 2010;42:391–404.
Bödeker ITM, Clemmensen KE, de Boer W, Martin F, Olson Å, Lindahl BD. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014;203:245–56.
Lindahl BD, Tunlid A. Ectomycorrhizal fungi–potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015;205:1443–7.
Šnajdr J, Valášková V, Merhautová V, Herinková J, Cajthaml T, Baldrian P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol Biochem. 2008;40:2068–75.
Kirk TK, Farrell RL. Enzymatic combustion: the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505.
Rineau F, Shah F, Smits MM, Persson P, Johansson T, Carleer R, et al. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 2013;7:2010–22.
Acknowledgements
Bengt Söderström and Gösta Hedberg are acknowledged for providing the study site, Olga Vinnere-Pettersson at SciLifeLab for advice on sequencing, Mikael Brandström-Durling for assistance with sequencing analysis and David Wardle, for commenting on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The work behind this manuscript was supported by The Swedish Research Council FORMAS, grants 2007-1365 and 2011-1747 to BDL. The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sterkenburg, E., Clemmensen, K.E., Ekblad, A. et al. Contrasting effects of ectomycorrhizal fungi on early and late stage decomposition in a boreal forest. ISME J 12, 2187–2197 (2018). https://doi.org/10.1038/s41396-018-0181-2
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41396-018-0181-2
This article is cited by
-
Tree species mixing causes shifts in nitrogen acquisition and utilization strategies of Mongolian pine and Simon poplar
Ecological Processes (2025)
-
Optimal rate of biochar application has positive effects on soil functional microbial abundance and agroecological function in a black soil of Northeast China
Carbon Letters (2025)
-
Ectomycorrhizal plant-mediated carbon fixation and microbial mechanisms of organic carbon stabilization in coastal saline soils
Journal of Soils and Sediments (2025)
-
Rhizosphere fungal diversity and plant-fungi interaction surpass bacteria in predicting soil multifunctionality during restoration in the Tengger desert
Journal of Soils and Sediments (2025)
-
Pervasive associations between dark septate endophytic fungi with tree root and soil microbiomes across Europe
Nature Communications (2024)