Abstract
Ostreococcus tauri, a picoeukaryotic alga that contributes significantly to primary production in oligotrophic waters, has a highly streamlined genome, lacking the genetic capacity to grow without the vitamins thiamine (B1) and cobalamin (B12). Here we demonstrate that the B12 and B1 auxotrophy of O. tauri can be alleviated by co-culturing with a heterotrophic bacterial partner Dinoroseobacter shibae, a member of the Rhodobacteraceae family of alpha-proteobacteria, genera of which are frequently found associated with marine algae. D. shibae lacks the complete pathway to synthesise three other B-vitamins: niacin (B3), biotin (B7), and p-aminobenzoic acid (a precursor for folate, B9), and the alga is in turn able to satisfy the reciprocal vitamin requirements of its bacterial partner in a stable long-term co-culture. Bioinformatics searches of 197 representative marine bacteria with sequenced genomes identified just nine species that had a similar combination of traits (ability to make vitamin B12, but missing one or more genes for niacin and biotin biosynthesis enzymes), all of which were from the Rhodobacteraceae. Further analysis of 70 species from this family revealed the majority encoded the B12 pathway, but only half were able to make niacin, and fewer than 13% biotin. These characteristics may have either contributed to or resulted from the tendency of members of this lineage to adopt lifestyles in close association with algae. This study provides a nuanced view of bacterial–phytoplankton interactions, emphasising the complexity of the sources, sinks and dynamic cycling between marine microbes of these important organic micronutrients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Li WKW. Primary production of prochlorophytes, cyanobacteria, and eukaryotic ultraphytoplankton: measurements from flow cytometric sorting. Limnol Oceanogr. 1992;39:169–75.
Countway PD, Caron DA. Abundance and distribution of Ostreococcus sp. in the San Pedro Channel, California, as revealed by quantitative PCR. Appl Environ Microbiol. 2006;72:2496–506.
Worden AZ, Nolan JK, Palenik B. Assessing the dynamics and ecology of marine picophytoplankton: The importance of the eukaryotic component. Limnol Oceanogr. 2004;49:168–79.
Massana R. Eukaryotic picoplankton in surface oceans. Annu Rev Microbiol. 2011;65:91–110.
Demir-Hilton E, Sudek S, Cuvelier ML, Gentemann CL, Zehr JP, Worden AZ. Global distribution patterns of distinct clades of the photosynthetic picoeukaryote Ostreococcus. ISME J. 2011;5:1095–107.
Derelle E, Ferraz C, Rombauts S, Rouzé P, Worden AZ, Robbens S, et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA. 2006;103:11647–52.
Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F. Vitamin B1 ecophysiology of marine picoeukaryotic algae: Strain-specific differences and a new role for bacteria in vitamin cycling. Limnol Oceanogr. 2015;60:215–28.
Helliwell KE, Wheeler GL, Leptos KC, Goldstein RE, Smith AG. Insights into the evolution of vitamin B12 auxotrophy from sequenced algal genomes. Mol Biol Evol. 2011;28:2921–33.
Bertrand EM, Allen AE. Influence of vitamin B auxotrophy on nitrogen metabolism in eukaryotic phytoplankton. Front Microbiol. 2012;3:375.
McRose D, Guo J, Monier A, Sudek S, Wilken S, Yan S, et al. Alternatives to vitamin B1 uptake revealed with discovery of riboswitches in multiple marine eukaryotic lineages. ISME J. 2014;8:2517–29.
Warren MJ, Raux E, Schubert HL, Escalante-Semerena JC. The biosynthesis of adenosylcobalamin (vitamin B12). Nat Prod Rep. 2002;19:390–412.
Croft MT, Lawrence AD, Raux-deery E, Warren MJ, Smith AG. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature. 2005;438:90–93.
Karl DM. Nutrient dynamics in the deep blue sea. Trends Microbiol. 2002;10:410–9.
Sañudo-Wilhelmy S, Cutter LS, Durazo R, Smail E, Gómez-Consarnau L, Webb E, et al. Multiple B-vitamin depletion in large areas of the coastal ocean. Proc Natl Acad Sci USA. 2012;109:14041–5.
Browning TJ, Achterberg EP, Rapp I, Engel A, Bertrand EM, Tagliabue A, et al. Nutrient co-limitation at the boundary of an oceanic gyre. Nature. 2017;551:242.
Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Kräutler B, et al. Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr Biol. 2016;26:999–1008.
Doxey AC, Kurtz DA, Lynch MD, Sauder LA, Neufeld JD. Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J. 2015;9:461–71.
Goecke F, Labes A, Wiese J, Imhoff JF. Phylogenetic analysis and antibiotic activity of bacteria isolated from the surface of two co-occurring macroalgae from the Baltic Sea. Eur J Phycol. 2013;48:47–60.
Cooper MB, Smith AG. Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr Opin Plant Biol. 2015;26:147–53.
Simon M, Scheuner C, Meier-Kolthoff JP, Brinkhoff T, Wagner-Döbler I, Ulbrich M, et al. Phylogenomics of Rhodobacteraceae reveals evolutionary adaptation to marine and non-marine habitats. ISME J. 2017;11:1483–99.
Biebl H, Allgaier M, Tindall BJ, Koblizek M, Lünsdorf H, Pukall R, et al. Dinoroseobacter shibae gen. nov., sp. nov., a new aerobic phototrophic bacterium isolated from dinoflagellates. Int J Syst Evol Microbiol. 2005;55:1089–96.
Wagner-Döbler I, Ballhausen B, Berger M, Brinkhoff T, Buchholz I, Bunk B, et al. The complete genome sequence of the algal symbiont Dinoroseobacter shibae: a hitchhiker’s guide to life in the sea. ISME J. 2010;4:61–77.
Durham BP, Sharma S, Luo H, Smith CB, Amin SA, Bender SJ, et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc Natl Acad Sci USA. 2015;112:453–7.
Amin SA, Hmelo LR, van Tol HM, Durham BP, Carlson LT, Heal KR, et al. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature. 2015;522:98–101.
Bertrand EM, McCrow JP, Moustafa A, Zheng H, McQuaid JB, Delmont TO, et al. Phytoplankton-bacterial interactions mediate micronutrient colimitation at the coastal Antarctic sea ice edge. Proc Natl Acad Sci USA. 2015;112:9938–43.
Lima-Mendez G, Faust K, Henry N, Decelle J, Colin S, Carcillo F, et al. Determinants of community structure in the global plankton interactome. Science. 2015;348:1262073–3.
Giovannoni SJ. Vitamins in the sea. Proc Natl Acad Sci USA. 2012;109:13888–9.
Helliwell KE, Pandhal J, Cooper MB, Longworth J, Kudahl UJ, Russo DA, et al. Quantitative proteomics of a B12 -dependent alga grown in coculture with bacteria reveals metabolic tradeoffs required for mutualism. New Phytol. 2018;217:599–612.
Poretsky RS, Hewson I, Sun S, Allen AE, Zehr JP, Moran MA. Comparative day/night metatranscriptomic analysis of microbial communities in the North Pacific subtropical gyre. Environ Microbiol. 2009;11:1358–75.
Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, eds. Nucleic Acid Techniques in Bacterial Systematic. New York, NY: John Wiley and Sons; 1991. p. 116–75.
Pace NR. A molecular view of microbial diversity and the biosphere. Science. 1997;276:740–3
Guillard RRL, Hargreaves PE. Stichochrysis immobilis is a diatom, not a crysophyte. Phycologia. 1993;32:234–6.
Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques. 1997;23:648–50.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8.
Gerdes S, Lerma-Ortiz C, Frelin O, Seaver SMD, Henry CS, de Crecy-Lagard V, et al. Plant B vitamin pathways and their compartmentation: a guide for the perplexed. J Exp Bot. 2012;63:5379–95.
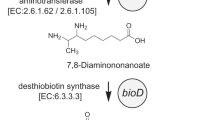
Webster SP, Alexeev D, Campopiano DJ, Watt RM, Alexeeva M, Sawyer L, et al. Mechanism of 8-amino-7-oxononanoate synthase: spectroscopic, kinetic, and crystallographic studies. Biochemistry. 2000;39:516–28.
Stoner GL, Eisenberg MA. Purification and properties of 7, 8-diaminopelargonic acid aminotransferase. J Biol Chem. 1975;250:4029–36.
Krell K, Eisenberg MA. The purification and properties of dethiobiotin synthetase. J Biol Chem. 1970;245:6558–66.
Berkovitch F, Nicolet Y, Wan JT, Jarrett JT, Drennan CL. Crystal structure of biotin synthase, an S-adenosylmethionine-dependent radical enzyme. Science. 2004;303:76–9.
Muralla R, Chen E, Sweeney C, Gray JA, Dickerman A, Nikolau BJ, et al. A bifunctional locus (BIO3-BIO1) required for biotin biosynthesis in arabidopsis. Plant Physiol. 2007;146:60–73.
Roje S. Vitamin B biosynthesis in plants. Phytochemistry. 2007;68:1904–21.
Yang Z, Savchenko A, Yakunin A, Zhang R, Edwards A, Arrowsmith C, et al. Aspartate dehydrogenase, a novel enzyme identified from structural and functional studies of TM1643. J Biol Chem. 2003;278:8804–8.
Green JM, Matthews RG. (2007). FolateBiosynthesis, reduction, and polyglutamylation and the interconversion of folate derivatives. EcoSal Plus. 2007;2. https://doi.org/10.1128/ecosalplus.3.6.3.6
Viswanathan VK, Green JM, Nichols BP. Kinetic characterization of 4-amino 4-deoxychorismate synthase from Escherichia coli. J Bacteriol. 1995;177:5918–23.
Camara D, Richefeu-Contesto C, Gambonnet B, Dumas R, Rébeillé F. The synthesis of pABA: Coupling between the glutamine amidotransferase and aminodeoxychorismate synthase domains of the bifunctional aminodeoxychorismate synthase from Arabidopsis thaliana. Arch Biochem Biophys. 2011;505:83–90.
Nakai T, Mizutani H, Miyahara I, Hirotsu K, Takeda S, Jhee KH, et al. Three-dimensional structure of 4-amino-4-deoxychorismate lyase from Escherichia coli. J Biochem. 2000;128:29–38.
Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–9.
Bertrand EM, Allen AE, Dupont CL, Norden-Krichmar TM, Bai J, Valas RE, et al. Cobalamin acquisition protein from diatoms. PNAS. 2012;109:E1762–71.
Rodriguez RJ, Freeman CD, McArthur DE, Kim YO, Redman RS. Symbiotic regulation of plant growth, development and reproduction. Commun Integr Biol. 2009;2:1–3.
Horvath B, Bachem CWB, Schell J, Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987;6:841–8.
Kazamia E, Czesnick H, Nguyen TTV, Croft MT, Sherwood E, Sasso S, et al. Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria exhibit regulation. Environ Microbiol. 2012;14:1466–76.
Amin SA, Parker MS, Armbrust EV. Interactions between diatoms and bacteria. Microbiol Mol Biol Rev. 2012;76:667–84.
Mukherjee S, Stamatis D, Bertsch J, Ovchinnikova G, Verezemska O, Isbandi M, et al. Genomes OnLine Database (GOLD) v.6: data updates and feature enhancements. Nucleic Acids Res. 2017;45:D446–56.
Flynn KJ, Stoecker DK, Mitra A, Raven JA, Glibert PM, Hansen PJ, et al. Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J Plankton Res. 2013;35:3–11.
Kazamia E, Helliwell KE, Purton S, Smith AG. How mutualisms arise in phytoplankton communities: building eco-evolutionary principles for aquatic microbes Fussmann G (ed). Ecol Lett. 2016;19:810–22.
Helliwell KE. The roles of B-vitamins in phytoplankton nutrition: new perspectives and prospects. New Phytol. 2017;216:62–68.
Natarajan KV. Distribution of thiamine, biotin, and niacin in the sea. Appl Microbiol. 1968;16:366–9.
Ford JE, Perry KD, Briggs CA. Nutrition of lactic acid bacteria isolated from the rumen. J Gen Microbiol. 1958;18:273–84.
Prunier AL, Schuch R, Fernández RE, Mumy KL, Kohler H, McCormick BA, et al. nadA and nadB of Shigella flexneri 5a are antivirulence loci responsible for the synthesis of quinolinate, a small molecule inhibitor of Shigella pathogenicity. Microbiology. 2007;153:2363–72.
Opitz CA, Heiland I. Dynamics of NAD-metabolism: everything but constant. Biochem Soc Trans. 2015;43:1127–32.
Tang YZ, Koch F, Gobler CJ. Most harmful algal bloom species are vitamin B-1 and B-12 auxotrophs. Proc Natl Acad Sci USA. 2010;107:20756–61.
Parniske M. Intracellular accommodation of microbes by plants: a common developmental program for symbiosis and disease? Curr Opin Plant Biol. 2000;3:320–8.
Seyedsayamdost MR, Case RJ, Kolter R, Clardy J. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat Chem. 2011;3:331–5.
Agosta SJ, Klemens JA. Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol Lett. 2008;11:1123–34.
Hom EFY, Murray AW. Plant-fungal ecology. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science. 2014;345:94–98.
Campbell K, Vowinckel J, Mülleder M, Malmsheimer S, Lawrence N, Calvani E, et al. Self-establishing communities enable cooperative metabolite exchange in a eukaryote. Elife. 2015;4:e09943.
Helliwell KE, Wheeler GL, Smith AG. Widespread decay of vitamin-related pathways: coincidence or consequence? Trends Genet. 2013;29:469–78.
Morris JJ, Lenski RE, Zinser ER. (2012) The Black Queen Hypothesis: evolution of dependencies through adaptive gene loss. mBio. 3;pii: e00036-12.
Acknowledgements
We thank Herve Moreau at Banyuls-sur-mer Oceanological Observatory in France for the gift of O. tauri OTH95. We are grateful to Nigel Miller (Department of Pathology, University of Cambridge) for help with FACS. We acknowledge funding from BBSRC grant (BB/I013164/1) and EU FP7 DEMA (Project no. 309086). MBC was in recipient of a CASE studentship with the UK Biotechnology and Biological Sciences Research Council (BBSRC) and Plymouth Marine Laboratory Applications Ltd, UJK was supported by EU FP7 Marie Curie ITN Photo.Comm (Project No 317184), and AS by the Cambridge BBSRC Doctoral Training Partnership.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cooper, M.B., Kazamia, E., Helliwell, K.E. et al. Cross-exchange of B-vitamins underpins a mutualistic interaction between Ostreococcus tauri and Dinoroseobacter shibae. ISME J 13, 334–345 (2019). https://doi.org/10.1038/s41396-018-0274-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-018-0274-y
This article is cited by
-
Unveiling plasmid diversity and functionality in pristine groundwater
Environmental Microbiome (2025)
-
Algal-bacterial symbiotic system for treatment of heavy metal containing wastewater: performance, mechanisms and applications
npj Clean Water (2025)
-
Disentangling the feedback loops driving spatial patterning in microbial communities
npj Biofilms and Microbiomes (2025)
-
Identifying potential keystone bacterial species within the phycosphere of marine algae and unveiling their metabolic characteristics
Marine Life Science & Technology (2025)
-
Symbiodiniaceae-Roseibium exhibit synergistic growth effects without B12 supplementation
Discover Oceans (2025)