Abstract
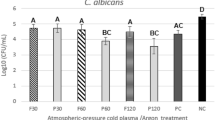
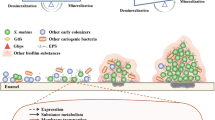
Candida albicans has been detected in root carious lesions. The current study aimed to explore the action of this fungal species on the microbial ecology and the pathogenesis of root caries. Here, by analyzing C. albicans in supragingival dental plaque collected from root carious lesions and sound root surfaces of root-caries subjects as well as caries-free individuals, we observed significantly increased colonization of C. albicans in root carious lesions. Further in vitro and animal studies showed that C. albicans colonization increased the cariogenicity of oral biofilm by altering its microbial ecology, leading to a polymicrobial biofilm with enhanced acidogenicity, and consequently exacerbated tooth demineralization and carious lesion severity. More importantly, we demonstrated that the cariogenicity-promoting activity of C. albicans was dependent on PHR2. Deletion of PHR2 restored microbial equilibrium and led to a less cariogenic biofilm as demonstrated by in vitro artificial caries model or in vivo root-caries rat model. Our data indicate the critical role of C. albicans infection in the occurrence of root caries. PHR2 is the major factor that determines the ecological impact and caries-promoting activity of C. albicans in a mixed microbial consortium.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Microbiome sequencing data have been deposited in public database Sequence Read Archive (http://www.ncbi.nlm.nih.gov/Traces/sra) with accession no. PRJNA613946. All data sets generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210.
de Olivera Carrilho MR. Root caries: from prevalence to therapy. Karger Medical and Scientific Publishers: Basel, 2017.
Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, et al. Dental caries. Nat Rev Dis Prim. 2017;3:17030.
Kreth J, Merritt J, Shi W, Qi F. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol. 2005;187:7193–203.
Caufield PW, Dasanayake AP, Li Y, Pan Y, Hsu J, Hardin JM. Natural history of Streptococcus sanguinis in the oral cavity of infants: evidence for a discrete window of infectivity. Infect Immun. 2000;68:4018–23.
Mikx FH, van der Hoeven JS, Plasschaert AJ, Konig KG. Establishment and symbiosis of Actinomyces viscosus, Streptococcus sanguis and Streptococcus mutans in germ-free Osborne-Mendel rats. Caries Res. 1976;10:123–32.
Mitrakul K, Vongsawan K, Sriutai A, Thosathan W. Association between S. mutans and S. sanguinis in severe early childhood caries and caries-free children a quantitative real-time PCR analysis. J Clin Pediatr Dent. 2016;40:281–9.
Loesche W, Rowan J, Straffon L, Loos P. Association of Streptococcus mutans with human dental decay. Infect Immun. 1975;11:1252–60.
Chen L, Qin B, Du M, Zhong H, Xu Q, Li Y, et al. Extensive description and comparison of human supra-gingival microbiome in root caries and health. PLoS One. 2015;10:e0117064.
Simon-Soro A, Guillen-Navarro M, Mira A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J Oral Microbiol. 2014;6:25443.
Babatzia A, Papaioannou W, Stavropoulou A, Pandis N, Kanaka-Gantenbein C, Papagiannoulis L, et al. Clinical and microbial oral health status in children and adolescents with type 1 diabetes mellitus. Int Dent J. 2020;70:136–44.
Zomorodian K, Kavoosi F, Pishdad GR, Mehriar P, Ebrahimi H, Bandegani A, et al. Prevalence of oral Candida colonization in patients with diabetes mellitus. J Mycol Med. 2016;26:103–10.
Mun M, Yap T, Alnuaimi AD, Adams GG, McCullough MJ. Oral candidal carriage in asymptomatic patients. Aust Dent J. 2016;61:190–5.
Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: a disease of opportunity. J Fungi. 2020;6:15.
Diaz PI, Hong BY, Dupuy AK, Strausbaugh LD. Mining the oral mycobiome: methods, components, and meaning. Virulence. 2017;8:313–23.
Shay K, Truhlar MR, Renner RP. Oropharyngeal candidosis in the older patient. J Am Geriatr Soc. 1997;45:863–70.
Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–8.
Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–35.
Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–71.
Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett. 2009;299:1–8.
Bamford CV, d’Mello A, Nobbs AH, Dutton LC, Vickerman MM, Jenkinson HF. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect Immun. 2009;77:3696–704.
Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010;6:e1000886.
Diaz PI, Xie Z, Sobue T, Thompson A, Biyikoglu B, Ricker A, et al. Synergistic interaction between Candida albicans and commensal oral streptococci in a novel in vitro mucosal model. Infect Immun. 2012;80:620–32.
Koo H, Andes DR, Krysan DJ. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018;14:e1007342.
Xu H, Sobue T, Thompson A, Xie Z, Poon K, Ricker A, et al. Streptococcal co-infection augments Candida pathogenicity by amplifying the mucosal inflammatory response. Cell Microbiol. 2014;16:214–31.
Xu H, Sobue T, Bertolini M, Thompson A, Dongari-Bagtzoglou A. Streptococcus oralis and Candida albicans synergistically activate mu-calpain to degrade E-cadherin from oral epithelial junctions. J Infect Dis. 2016;214:925–34.
Xu H, Sobue T, Bertolini M, Thompson A, Vickerman M, Nobile CJ, et al. S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms. Virulence. 2017;8:1602–17.
Lozano Moraga CP, Rodriguez Martinez GA, Lefimil Puente CA, Morales Bozo IC, Urzua, Orellana BR. Prevalence of Candida albicans and carriage of Candida non-albicans in the saliva of preschool children, according to their caries status. Acta Odontol Scand. 2017;75:30–5.
Wu N, Lin J, Wu L, Zhao J. Distribution of Candida albicans in the oral cavity of children aged 3-5 years of Uygur and Han nationality and their genotype in caries-active groups. Genet Mol Res. 2015;14:748–57.
Xiao J, Moon Y, Li L, Rustchenko E, Wakabayashi H, Zhao X, et al. Candida albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PLoS One. 2016;11:e0164242.
Xiao J, Grier A, Faustoferri RC, Alzoubi S, Gill AL, Feng C, et al. Association between oral candida and bacteriome in children with severe ECC. J Dent Res. 2018;97:1468–76.
Xiao J, Huang X, Alkhers N, Alzamil H, Alzoubi S, Wu TT, et al. Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 2018;52:102–12.
Falsetta ML, Klein MI, Colonne PM, Scott-Anne K, Gregoire S, Pai CH, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82:1968–81.
Beighton D, Lynch E. Relationships between yeasts and primary root-caries lesions. Gerodontology. 1993;10:105–8.
Shen S, Samaranayake LP, Yip HK. Coaggregation profiles of the microflora from root surface caries lesions. Arch Oral Biol. 2005;50:23–32.
Zaremba ML, Stokowska W, Klimiuk A, Daniluk T, Rozkiewicz D, Cylwik-Rokicka D, et al. Microorganisms in root carious lesions in adults. Adv Med Sci. 2006;51 Suppl 1:237–40.
Dige I, Nyvad B. Candida species in intact in vivo biofilm from carious lesions. Arch Oral Biol. 2019;101:142–6.
Ev LD, Dame-Teixeira N, Do T, Maltz M, Parolo CCF. The role of Candida albicans in root caries biofilms: an RNA-seq analysis. J Appl Oral Sci. 2020;28:e20190578.
Edlund A, Yang Y, Hall AP, Guo L, Lux R, He X, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1:25.
Tian Y, He X, Torralba M, Yooseph S, Nelson KE, Lux R, et al. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol. 2010;25:357–67.
Zhang K, Ren B, Zhou X, Xu HH, Chen Y, Han Q, et al. Effect of antimicrobial denture base resin on multi-species biofilm formation. Int J Mol Sci. 2016;17:e1033.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21.
Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–8.
Klug B, Rodler C, Koller M, Wimmer G, Kessler HH, Grube M, et al. Oral biofilm analysis of palatal expanders by fluorescence in-situ hybridization and confocal laser scanning microscopy. J Vis Exp. 2011;56:e2967–e.
Xiao J, Klein MI, Falsetta ML, Lu B, Delahunty CM, Yates JR III, et al. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012;8:e1002623.
Du Q, Fu M, Zhou Y, Cao Y, Guo T, Zhou Z, et al. Sucrose promotes caries progression by disrupting the microecological balance in oral biofilms: an in vitro study. Sci Rep. 2020;10:2961.
Firestone AR, Graves CN, Feagin FF. The effects of different levels of dietary sucrose on root caries subsequent to gingivectomy in conventional rats infected with Actinomyces viscosus M-100. J Dent Res. 1988;67:1342–5.
Doff RS, Rosen S, App G. Root surface caries in the molar teeth of Rice rats. I. A method for quantitative scoring. J Dent Res. 1977;56:1013–6.
Xu X, Chen F, Huang Z, Ma L, Chen L, Pan Y, et al. Meeting report: a close look at oral biofilms and microbiomes. Int J Oral Sci. 2018;10:28.
Lambooij JM, Hoogenkamp MA, Brandt BW, Janus MM, Krom BP. Fungal mitochondrial oxygen consumption induces the growth of strict anaerobic bacteria. Fungal Genet Biol. 2017;109:1–6.
Janus MM, Crielaard W, Volgenant CM, van der Veen MH, Brandt BW, Krom BP. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J Oral Microbiol. 2017;9:1270613.
Delaney C, O’Donnell LE, Kean R, Sherry L, Brown JL, Calvert G, et al. Interkingdom interactions on the denture surface: implications for oral hygiene. Biofilm. 2019;1:100002.
Hwang G, Marsh G, Gao L, Waugh R, Koo H. Binding force dynamics of Streptococcus mutans-glucosyltransferase B to Candida albicans. J Dent Res. 2015;94:1310–7.
Gregoire S, Xiao J, Silva BB, Gonzalez I, Agidi PS, Klein MI, et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl Environ Microbiol. 2011;77:6357–67.
Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86.
Hwang G, Liu Y, Kim D, Li Y, Krysan DJ, Koo H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017;13:e1006407.
Kim D, Koo H. Spatial design of polymicrobial oral biofilm in its native disease state. J Dent Res. 2020;99:597–603.
Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–42.
Kim D, Liu Y, Benhamou RI, Sanchez H, Simon-Soro A, Li Y, et al. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018;12:1427–42.
Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, et al. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–38.
Kim D, Sengupta A, Niepa TH, Lee BH, Weljie A, Freitas-Blanco VS, et al. Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci Rep. 2017;7:41332.
Sztajer H, Szafranski SP, Tomasch J, Reck M, Nimtz M, Rohde M, et al. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014;8:2256–71.
Rossi DCP, Gleason JE, Sanchez H, Schatzman SS, Culbertson EM, Johnson CJ, et al. Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis. PLoS Pathog. 2017;13:e1006763.
Ellepola K, Truong T, Liu Y, Lin Q, Lim TK, Lee YM, et al. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans–Candida albicans mixed-species biofilms. Infect Immun. 2019;87:e00339–19.
Khoury ZH, Vila T, Puthran TR, Sultan AS, Montelongo-Jauregui D, Melo MAS, et al. The role of Candida albicans secreted polysaccharides in augmenting Streptococcus mutans adherence and mixed biofilm formation: in vitro and in vivo studies. Front Microbiol. 2020;11:307.
Baek YU, Martin SJ, Davis DA. Evidence for novel pH-dependent regulation of Candida albicans Rim101, a direct transcriptional repressor of the cell wall beta-glycosidase Phr2. Eukaryot Cell. 2006;5:1550–9.
Muhlschlegel FA, Fonzi WA. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol Cell Biol. 1997;17:5960–7.
Saporito-Irwin SM, Birse CE, Sypherd PS, Fonzi WA. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–13.
De Bernardis F, Muhlschlegel FA, Cassone A, Fonzi WA. The pH of the host niche controls gene expression in and virulence of Candida albicans. Infect Immun. 1998;66:3317–25.
Calderon J, Zavrel M, Ragni E, Fonzi WA, Rupp S, Popolo L. PHR1, a pH-regulated gene of Candida albicans encoding a glucan-remodelling enzyme, is required for adhesion and invasion. Microbiology. 2010;156:2484–94.
Acknowledgements
We greatly thank Professors Williama A. Fonzi, Dominique Sanglard, Aaron P. Mitchell, David Kadosh, Joachim F. Ernst, Gerald R. Fink, Haoping Liu and Lo Hsiu-Jung for kindly providing fungal strains. Vivian Chen is a recipient of a 2017–2018 Fulbright Research grant to China. This work was supported by the National Natural Science Foundation of China (81771099 to XX, 81870754 to XZ, 81600858 to BR, 31720103901 to Lixin Zhang), the “111” Project of China (B18022 to Lixin Zhang), a research grant from the Department of Science & Technology Sichuan Province (2018SZ0121 to XX), and the Fundamental Research Funds for the Central Universities (22221818014S to Lixin Zhang).
Author information
Authors and Affiliations
Contributions
Conceptualization: QD, BR, L. Zhang, XZ, and XX; methodology: QD, BR, L. Zhang, XZ, and XX; formal analysis: QD, JH, QG, and L. Zheng; investigation: QD, JH, XP, QG; resources: XP, QG, L. Zheng; data curation: JL, HD; writing original draft: QD, BR, XZ, XX; writing, review, and editing: VC, L. Zhang, XZ, XX; visualization: QD, BR, XX; supervision: L. Zhang, XZ; funding acquisition: BR, XZ, XX.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Du, Q., Ren, B., He, J. et al. Candida albicans promotes tooth decay by inducing oral microbial dysbiosis. ISME J 15, 894–908 (2021). https://doi.org/10.1038/s41396-020-00823-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-020-00823-8
This article is cited by
-
In vitro evaluation of tolerance ability of cross-kingdom biofilm towards oral dynamic fluctuations
BMC Oral Health (2025)
-
D-lactic acid inhibits Candida albicans associated with vulvovaginal candidiasis: in vitro and in vivo effects and underlying mechanisms
BMC Microbiology (2025)
-
Essential oils enriched Dant-Kanti-Gandush (oil-pulling) inhibits inter-kingdom biofilm formation on orthodontic fixtures and ameliorates cariogenic virulence factors of oral pathogens
Scientific Reports (2025)
-
Effect of host microenvironment and bacterial lifestyles on antimicrobial sensitivity and implications for susceptibility testing
npj Antimicrobials and Resistance (2025)
-
The landscape of the microbiome at different stages of root caries
Clinical Oral Investigations (2025)