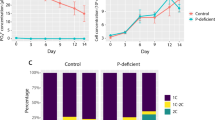
Abstract
Adaptation of cell populations to environmental changes is mediated by phenotypic variability at the single-cell level. Enzyme activity is a key factor in cell phenotype and the expression of the alkaline phosphatase activity (APA) is a fundamental phytoplankton strategy for maintaining growth under phosphate-limited conditions. Our aim was to compare the APA among cells and species revived from sediments of the Bay of Brest (Brittany, France), corresponding to a pre-eutrophication period (1940’s) and a beginning of a post-eutrophication period (1990’s) during which phosphate concentrations have undergone substantial variations. Both toxic marine dinoflagellate Alexandrium minutum and the non-toxic dinoflagellate Scrippsiella acuminata were revived from ancient sediments. Using microfluidics, we measured the kinetics of APA at the single-cell level. Our results indicate that all S. acuminata strains had significantly higher APA than A. minutum strains. For both species, the APA in the 1990’s decade was significantly lower than in the 1940’s. For the first time, our results reveal both inter and intraspecific variabilities of dinoflagellate APA and suggest that, at a half-century timescale, two different species of dinoflagellate may have undergone similar adaptative evolution to face environmental changes and acquire ecological advantages.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gobler CJ, Doherty OM, Hattenrath-Lehmann TK, Griffith AW, Kang R, Litaker W. Ocean warming has expanded niche of toxic algae. Proc Natl Acad Sci USA. 2017;114:4975–80.
Olivieri ET. Colonization, adaptations and temporal changes in diversity and biomass of a phytoplankton community in upwelled water off the Cape Peninsula, South Africa, in December 1979. South Afr J Mar Sci. 1983;1:77–109.
Irwin AJ, Zoe V, Finkel ZV, Müller-Karger FE, Troccoli, Ghinaglia L. Phytoplankton adapt to changing ocean environment. Proc Natl Acad Sci USA. 2015;112:5762–6.
Chivers W, Walne A, Hays G. Mismatch between marine plankton range movements and the velocity of climate change. Nat Commun. 2017;8:14434.
Gisselson L, Granéli E, Pallon J. Variation in cellular nutrient status within a population of Dinophysis norvegica (Dinophyceae) growing in situ: single - cell elemental analysis by use of a nuclear microprobe. Limnol Oceanogr. 2001;5. https://doi.org/10.4319/lo.2001.46.5.1237.
Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. https://doi.org/10.1038/nrmicro3491.
Núñez-Milland DR, Baines SB, Vogt S, Twining BS. Quantification of phosphorus in single cells using synchrotron X-ray fluorescence. J Synchrotron Radiat. 2010;17:560–6.
Berthelot H, Duhamel S, L’Helguen S, Maguer JF, Wang S, Cetinić I, et al. NanoSIMS single cell analyses reveal the contrasting nitrogen sources for small phytoplankton. ISME J. 2019;13:651–62. https://doi.org/10.1038/s41396-018-0285-8.
Štrojsová A, Vrba J. Short-term variation in extracellular phosphatase activity: possible limitations for diagnosis of nutrient status in particular algal populations. Aquat Ecol. 2009;43:19–25.
O’Donnell DR, Hamman CR, Johnson EC, Kremer CT, Klausmeier CA, Litchman E. Rapid thermal adaptation in a marine diatom reveals constraints and trade-offs. Glob Change Biol. 2018;24:4554–65.
Jin P, Agustí S. Fast adaptation of tropical diatoms to increased warming with trade-offs. Sci Rep. 2018;8:17771. https://doi.org/10.1038/s41598-018-36091-y.
Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, et al. Extinction risk from climate change. Nature. 2004;427:145–8.
Urban MC. Accelerating extinction risk from climate change. Science. 2015;348:571–3.
Kottuparambil S, Jin P, Agusti S. Adaptation of Red Sea Phytoplankton to experimental warming increases their tolerance to toxic metal exposure. Front Environ Sci. 2019;7. https://doi.org/10.3389/fenvs.2019.00125.
Flores-Moya A, Costas E, Lopez-Rodas V. Roles of adaptation, chance and history in the evolution of the dinoflagellate Prorocentrum triestinum. Naturwissenschaften. 2008;95:697–703.
Flores-Moya A, Rouco M, García-Sánchez MJ, García-Balboa C, González R, Costas E, et al. Effects of adaptation, chance, and history on the evolution of the toxic dinoflagellate Alexandrium minutum under selection of increased temperature and acidification. Ecol Evol. 2012;2:1251–9. https://doi.org/10.1002/ece3.198.
Martiny AC, Ustick LA, Garcia C, Lomas MW. Genomic adaptation of marine phytoplankton populations regulates phosphate uptake. Limnol Oceanogr. 2019. https://doi.org/10.1002/lno.11252.
Ribeiro S, Berge T, Lundholm N, Andersen TJ, Abrantes F, Ellegaard M. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat Commun. 2011;2:311.
Delebecq G, Schmidt S, Ehrhold A, Latimier M, Siano R. Revival of ancient marine dinoflagellates using molecular biostimulation. J Phycol. 2020;56:1077–89.
Ribeiro S, Berge T, Lundholm N, Ellegaard M. Hundred years of environmental change and phytoplankton ecophysiological variability archived in coastal sediments. PLoS ONE. 2013;8:e61184.
Klouch KZ, Schmidt S, Andrieux Loyer F, Le Gac M, Hervio-Heath D, Qui-Minet ZN, et al. Historical records from dated sediment cores reveal the multidecadal dynamic of the toxic dinoflagellate Alexandrium minutum in the Bay of Brest (France). FEMS Microbiol Ecol. 2016;92:1–16.
Lundholm N, Ribeiro S, Godhe A, Rostgaard Nielsen L, Ellegaard M. Exploring the impact of multidecadal environmental changes on the population genetic structure of a marine primary producer. Ecol Evol. 2017;7:3132–42.
Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW, et al. Processes and patterns of oceanic nutrient limitation. Nat Geosci. 2013;6:701–10.
Labry C, Herbland A, Delmas D. The role of phosphorus on planktonic production of the Gironde plume waters in the Bay of Biscay. J Plankt Res. 2002;24:97–117.
Girault M, Arakawa H, Hashihama F. Phosphorus stress of microphytoplankton community in the western subtropical North Pacific. J Plankt Res. 2013;35:146–57.
Ramos JBE, Schulz KG, Voss M, Narciso Á, Müller MN, Reis FV, et al. Nutrient-specific responses of a phytoplankton community: a case study of the North Atlantic Gyre. Azores J Plankt Res. 2017;39:744–61.
Lin S, Litaker RW, Sunda WG. Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J Phycol. 2016;52:10–36.
Lomas MW, Swain A, Shelton R, Ammerman JW. Taxonomic variability of phosphorus stress in Sargasso Sea phytoplankton. Limnol Oceanogr. 2004;49:2303–10.
Wang D, Huang B, Liu X, Liu G, Wang H. Seasonal variations of phytoplankton phosphorus stress in the Yellow Sea Cold Water Mass. Acta Oceano Sin. 2014;33:124–35.
Cembella AD, Antia NJ, Harrison PJ. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: part I. CRC Crit Rev Microbiol. 1984;10:317–91.
Cooper A, Bowen ID, Lloyd D. The properties and subcellular localization of acid phosphatases in the colourless alga Polytomella caeca. J Cell Sci. 1974;15:605–18.
Duhamel S, Björkman KM, Van Wambeke F, Moutin T, Karl DM. Characterization of alkaline phosphatase activity in the North and South Pacific Subtropical Gyres: Implications for phosphorus cycling. Limnol Oceanogr. 2011;56:1244–54.
Kang W, Wang ZH, Liu L, Guo X. Alkaline phosphatase activity in the phosphorus-limited southern Chinese coastal waters. J Environ Sci. 2019;86:38–49.
Girault M, Beneyton T, Pekin D, Buisson L, Bichon S, Charbonnier C, et al. High-content screening of plankton alkaline phosphatase activity in microfluidics. Anal Chem. 2018;90:4174–81. https://doi.org/10.1021/acs.analchem.8b00234.
Anderson RA, Berges RA, Harrison PJ, Watanabe MM. Appendix A - recipes for freshwater and seawater media; enriched natural seawater media. In Andersen RA, editor. Algal culturing techniques. San Diego, USA: Academic; 2005. p. 429–538.
Guillard RL, Ryther JH. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962;8:229–39.
Duffy DC, McDonald JC, Schueller OJ, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem. 1998;70:4974–84.
Girault M, Hattori A, Kim H, Arakawa H, Matsuura K, Odaka M, et al. An on-chip imaging droplet-sorting system: a real-time shape recognition method to screen target cells in droplets with single cell resolution. Sci Rep. 2017;7:40072. https://doi.org/10.1038/srep40072.
Girault M, Odaka M, Kim H, Matsuura K, Terazono H, Yasuda K. Particle recognition in microfluidic applications using a template matching algorithm. JPN J Appl Phys. 2016;55. https://doi.org/10.7567/JJAP.55.06GN05.
Urvoy M, Labry C, Delmas D, Creac’h L, L’Helguen S. Microbial enzymatic assays in environmental water samples: impact of Inner Filter Effect and substrate concentrations. Limnol Oceanogr Methods. 2020;18:728–38.
Huang Z, Terpetschnig E, You W, Haugland RP. 2-(2′-phosphoryloxyphenyl)-4-(3H)-quinazolinone derivatives as fluorogenic precipitating substrates of phosphatases. Anal Biochem. 1992;207:32–39.
Girault M, Hattori A, Kim H, Matsuura K, Odaka M, Terazono H et al. Algorithm for the precise detection of single and cluster cells in microfluidic applications. Cytom Part A. 2016. https://doi.org/10.1002/cyto.a.22825.
Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36.
Hoppe HG. Phosphatase activity in the sea. Hydrobiologia. 2003;493:187–200.
Golda-VanEeckhoutte RL, Roof LT, Needoba JA, Peterson DT. Determination of intracellular pH in phytoplankton using the fluorescent probe, SNARF, with detection by fluorescence spectroscopy. J Microbiol Methods. 2018;152:109–18.
Kruskopf MM, Du Plessis S. Induction of both acid and alkaline phosphatase activity in two green-algae (chlorophyceae) in low N and P concentrations. Hydrobiologia. 2004;513:59–70.
Štrojsová A, Vrba J, Nedoma J, Komárková J, Znachor P. Seasonal study of extracellular phosphatase expression in the phytoplankton of a eutrophic reservoir. Eur J Phycol. 2003;38:295–306.
Skelton HM, Parrow MW, Burkholder JM. Phosphatase activity in the heterotrophic dinoflagellate Pfiesteria shumwayae. Harmful Algae 2006;5:395–406.
Nedoma J, Štrojsová A, Vrba J, Komárková J, Simek K. Extracellular phosphatase activity of natural plankton studied with ELF97 phosphate: fluorescence quantification and labelling kinetics. Environ Microbiol. 2003;5:462–72.
Young EB, Tucker RC, Pansch LA. Alkaline phosphatase in freshwater Cladophora-epiphyte assemblages: regulation in response to phosphorus supply and localization. J Phycol. 2010;46:93–101.
Díaz-de-Quijano D, Felip M. A comparative study of fluorescence-labelled enzyme activity methods for assaying phosphatase activity in phytoplankton. A possible bias in the enzymatic pathway estimations. J Micro Meth. 2011;86:104–7.
Ou L, Huang B, Lin L, Hong H, Zhang F, Chen Z. Phosphorus stress of phytoplankton in the Taiwan Strait determined by bulk and single-cell alkaline phosphatase activity assays. Mar Ecol Prog Ser. 2006;327:95–106.
Huang B, Ou L, Wang X, Huo W, Li R, Hong H, et al. Alkaline phosphatase activity of phytoplankton in East China Sea coastal waters with frequent harmful algal bloom occurrences. Aquat Micro Ecol. 2007;49:195–206.
Ivančić I, Pfannkuchen M, Godrijan J, Djakovac T, Pfannkuchen DM, Korlević M, et al. Alkaline phosphatase activity related to phosphorus stress of microphytoplankton in different trophic conditions. Prog Oceanogr. 2016;146:175–86.
González-Gil S, Keafer B, Jovine JMR, Aguileral A, Lu S, Anderson DM. Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Mar Ecol Prog Ser. 1998;164:21–35.
Dyhrman ST, Ruttenberg KC. Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: Implications for dissolved organic phosphorus remineralization. Limnol Oceanogr. 2006;51. https://doi.org/10.4319/lo.2006.51.3.1381.
Flynn K, Jones KJ, Flynn KJ. Comparisons among species of Alexandrium (Dinophyceae) grown in nitrogen- or phosphorus-limiting batch culture. Mar Biol. 1996;126:9–18.
Perry MJ. Alkaline phosphatase activity in subtropical Central North Pacific waters using a sensitive fluorometric method. Mar Biol. 1972;15:113–9.
Dyhrman ST, Palenik B. Phosphate stress in cultures and field populations of the dinoflagellate prorocentrum minimum detected by a single-cell alkaline phosphatase assay. Appl Environ Microbiol. 1999;65:3205–12.
Mulholland MR, Floge S, Carpenter EJ, Capone DG. Phosphorus dynamics in cultures and natural populations of Trichodesmium spp. Mar Ecol Prog Ser. 2002;239:45–55.
Thomson B, Wenley J, Currie K, Hepburn C, Herndl GJ, Baltar F. Resolving the paradox: Continuous cell-free alkaline phosphatase activity despite high phosphate concentrations. Mar Chem. 2019;214:103671.
Foster RA, Sztejrenszus S, Kuypers MMM. Measuring carbon and N2 fixation in field populations of colonial and free-living unicellular cyanobacteria using nanometer-scale secondary ion mass spectrometry. J Phycol. 2013;49:502–16.
Dyhrman ST, Palenik B. Characterization of ectoenzyme activity and phosphate-regulated proteins in the coccolithophorid Emiliania huxleyi. J Plankton Res. 2003;25:1215–25.
Oh SJ, Yamammoto T, Kataoka Y, Matsuda O, Matsuyama Y, Katani Y. Utilization of dissolved organic phosphorus by the two toxic dinoflagellates, Alexandrium tamarense and Gymnodinium catenatum (Dinophyceae). Fish Sci. 2002;68:416–24.
Jauzein C, Labry C, Youenou A, Quéré J, Delmas D, Collos Y. Growth and phosphorus uptake by the toxic dinoflagellate Alexandrium catenella (Dinophycea) in response to phosphate limitation. J Phycol. 2010;46:926–36.
Elgavish A, Halmann M, Berman T. A comparative study of phosphorus utilization and storage in batch cultures of Peridinium cinctum, Pediastrum duplex and Cosmarium sp., from Lake Kinneret (Israel). Phycologia. 1982;21:47–54.
Flynn K, Franco JM, Fernandez P, Reguera B, Zapata M, Wood G, et al. Changes in toxin content, biomass and pigments of the dinoflagellate Alexandrium minutum during nitrogen refeeding and growth into nitrogen or phosphorus stress. Mar Ecol Prog Ser. 1994;111:99–109.
Ou L, Wang D, Huang B, Hong H, Qi Y, Lu S. Comparative study of phosphorus strategies of three typical harmful algae in Chinese coastal waters. J Plankton Res. 2008;30:1007–17.
Droop MR. The nutrient status of algal cells in continuous culture. J Mar Biol Ass UK. 1974;54:825–55.
Bechemin C, Grzebyk D, Hachame F, Hummert C, Maestrini S. Effect of different nitrogen/phosphorus nutrient ratios on the toxin content in Alexandrium minutum. Aquat Micro Ecol. 1990;20:157–65.
Labry C, Erard–Le Denn E, Chapelle A, Fauchot J, Youenou A, Crassous MP, et al. Competition for phosphorus between two dinoflagellates: A toxic Alexandrium minutum and a non-toxic Heterocapsa triquetra. J Exp Mar Biol Ecol. 2008;358:124–35.
Chapelle A, Labry C, Sourisseau M, Lebreton C, Youenou A, Crassous MP. Alexandrium minutum growth controlled by phosphorus An applied model. J Mar Syst. 2010;83:181–91.
Sakshaug E, Granéli E, Elbrächter M, Kayser H. Chemical composition and alkaline phosphatase activity of nutrient-saturated and P-deficient cells of four marine dinoflagellates. J Exp Mar Biol Ecol. 1984;11:241–54.
Lirdwitayaprasit T, Okaichi T, Montani S, Ochi T, Anderson DM. Changes in cell chemical con~position during the life cycle of Scrippsiella trochoidea (Dinophyceae). J Phycol. 1990;26:299–306.
Qi H, Wang J, Wang Z. A comparative study of maximal quantum yield of photosystem II to determine nitrogen and phosphorus limitation on two marine algae. J Sea Res. 2013;80:1–11.
Simon N, Cras AL, Foulon E, Lemée R. Diversity and evolution of marine phytoplankton. C R Biol. 2009;332:159–70.
Rengefors K, Kremp A, Reusch TBH, Wood AM. Genetic diversity and evolution in eukaryotic phytoplankton: revelations from population genetic studies. J Plankton Res. 2017;39:165–79.
Bendif EM, Nevado B, Wong ELY, Wong EL, Hagino K, Probert I, et al. Repeated species radiations in the recent evolution of the key marine phytoplankton lineage Gephyrocapsa. Nat Commun. 2019;10:4234.
Thornton DCO. Individuals clones or groups? Phytoplankton behaviour and units of selection. Ethol Ecol Evol. 2002;14:165–73.
Gerecht A, Romano G, Lanora A, d’Ippolito G, Cutignano A, Fontana A. Plasticity of Oxylipin metabolism among clones of the marine diatom Skeletonema marinoi (Bacillariophyceae). J Phycol. 2011;47:1050–6.
Lim PT, Leaw CP, Usup G, Kobiyama A, Koike K, Ogata T. Effects of light and temperature on growth, nitrate uptake, and toxin production of two tropical dinoflagellates: Alexandrium tamiyavanichii and Alexandrium minutum (Dinophyceae). J Phycol. 2006;42:786–99.
Van Mooy BA, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature. 2009;458:69–72.
Galbraith AD, Martiny AC. Simple mechanism for marine nutrient stoichiometry. Proc Natl Acad Sci USA. 2015;112:8199–204.
Berge T, Daugbjerg N, Hansen PJ. Isolation and cultivation of microalgae select for low growth rate and tolerance to high pH. Harmful Algae. 2012;20:101–10.
Acknowledgements
We thank the Syndicat de bassin d’Elorn for providing the historical data of nitrate and phosphate concentrations. MG, JCB and YDA acknowledge financial support of a Marie-Curie Individual Fellowships (IF) MAPAPAIMA (797007). JCB acknowledges the financial support of the Region Nouvelle Aquitaine and from the French state in the frame of the ‘investment for the future’ (Programme IdEx Bordeaux, ANR-10-IDEX-03–02). Revived cultures and culture experiments were carried out in the frame of the project PALMIRA part of the project PALMIRA (Palaeoecology of Alexandrium minutum dans la Rade de Brest–Marché n°2017–90292) financed by the Région Brittany. We are grateful to all members of the crew of the N/O Thalia ship of Ifremer for providing technical expertise in sediment core collection and to Emulseo for providing the surfactant used in this study. We thank Prof. Josh D. Neufeld and anonymous reviewers for their valuable comments and insights that considerably helped to improve the manuscript.
Author information
Authors and Affiliations
Contributions
MG, JCB and RS conceived and designed the study (MG, JCB microfluidics; MG, RS microbiology). Experiments were performed by MG (APA experiments, microfluidics), CL (phosphate concentrations measurements) and ML (cultivation and preparation of cells) under the supervision of RS and JCB. MG, LB and TB contributed analytical tools (MG, LB microfluidic platform and instrumentation; MG, TB microfluidic chips, MG image processing). MG, CL, CJ, YDA and JCB contributed to data analysis. MG, JCB and RS wrote the manuscript with contributions from all authors.
Corresponding authors
Ethics declarations
Conflict of interest
JCB is a co-founder of Emulseo whose surfactant formulation was used in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Girault, M., Siano, R., Labry, C. et al. Variable inter and intraspecies alkaline phosphatase activity within single cells of revived dinoflagellates. ISME J 15, 2057–2069 (2021). https://doi.org/10.1038/s41396-021-00904-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-021-00904-2
This article is cited by
-
Extracellular enzymatic activities of octocorals and scleractinian corals under environmental stress
Scientific Reports (2025)
-
Reviving and characterizing three species of dinoflagellate cysts dormant for about 70 years in the East China Sea: Biecheleria brevisulcata, Biecheleriopsis adriatica, and Scrippsiella donghaienis
Journal of Oceanology and Limnology (2022)