Abstract
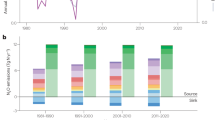
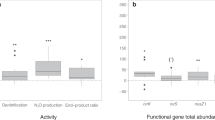
Nitrous oxide (N2O) is an important greenhouse gas and an ozone-depleting substance. Due to the long persistence of N2O in the atmosphere, the mitigation of anthropogenic N2O emissions, which are mainly derived from microbial N2O-producing processes, including nitrification and denitrification by bacteria, archaea, and fungi, in agricultural soils, is urgently necessary. Members of mesofauna affect microbial processes by consuming microbial biomass in soil. However, how microbial consumption affects N2O emissions is largely unknown. Here, we report the significant role of fungivorous mites, the major mesofaunal group in agricultural soils, in regulating N2O production by fungi, and the results can be applied to the mitigation of N2O emissions. We found that the application of coconut husks, which is the low-value part of coconut and is commonly employed as a soil conditioner in agriculture, to soil can supply a favorable habitat for fungivorous mites due to its porous structure and thereby increase the mite abundance in agricultural fields. Because mites rapidly consume fungal N2O producers in soil, the increase in mite abundance substantially decreases the N2O emissions from soil. Our findings might provide new insight into the mechanisms of soil N2O emissions and broaden the options for the mitigation of N2O emissions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Montzka SA, Dlugokencky EJ, Butler JH. Non-CO2 greenhouse gases and climate change. Nature. 2011;476:43–50.
Ravishankara A, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–5.
Pachauri RK, Reisinger A, Climate Change 2007. Synthesis report. Contribution of Working Groups I, II and III to the fourth assessment report. Cambridge: Cambridge University Press; 2008.
Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, et al. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. Geneva: World Meteorological Organisation; 2018.
Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, et al. Global agriculture and nitrous oxide emissions. Nat Clim Change. 2012;2:410–6.
Itakura M, Uchida Y, Akiyama H, Hoshino YT, Shimomura Y, Morimoto S, et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat Clim Change. 2013;3:208–12.
Akiyama H, Yan X, Yagi K. Evaluation of effectiveness of enhanced‐efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta‐analysis. Glob Change Biol. 2010;16:1837–46.
Hu HW, Trivedi P, He JZ, Singh BK. Microbial nitrous oxide emissions in dryland ecosystems: mechanisms, microbiome and mitigation. Environ Microbiol. 2017;19:4808–28.
Bakken LR, Frostegård Å. Sources and sinks for N2O, can microbiologist help to mitigate N2O emissions? Environ Microbiol. 2017;19:4801–5.
Paustian K, Lehmann J, Ogle S, Reay D, Robertson GP, Smith P. Climate-smart soils. Nature. 2016;532:49–57.
Wrage N, Velthof G, Van Beusichem M, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol Biochem. 2001;33:1723–32.
Shoun H, Kim D-H, Uchiyama H, Sugiyama J. Denitrification by fungi. FEMS Microbiol Lett. 1992;94:277–81.
Maeda K, Spor A, Edel-Hermann V, Heraud C, Breuil M-C, Bizouard F, et al. N2O production, a widespread trait in fungi. Sci Rep. 2015;5:1–7.
Mothapo NV, Chen H, Cubeta MA, Shi W. Nitrous oxide producing activity of diverse fungi from distinct agroecosystems. Soil Biol Biochem. 2013;66:94–101.
Wei W, Isobe K, Shiratori Y, Nishizawa T, Ohte N, Otsuka S, et al. N2O emission from cropland field soil through fungal denitrification after surface applications of organic fertilizer. Soil Biol Biochem. 2014;69:157–67.
Jirout J, Šimek M, Elhottová D. Fungal contribution to nitrous oxide emissions from cattle impacted soils. Chemosphere. 2013;90:565–72.
Laughlin RJ, Stevens RJ. Evidence for fungal dominance of denitrification and codenitrification in a grassland soil. Soil Sci Soc Am J. 2002;66:1540–8.
Blagodatskaya E, Dannenmann M, Gasche R, Butterbach-Bahl K. Microclimate and forest management alter fungal-to-bacterial ratio and N2O-emission during rewetting in the forest floor and mineral soil of mountainous beech forests. Biogeochemistry. 2010;97:55–70.
Wankel SD, Ziebis W, Buchwald C, Charoenpong C, de Beer D, Dentinger J, et al. Evidence for fungal and chemodenitrification based N2O flux from nitrogen impacted coastal sediments. Nat Commun. 2017;8:1–11.
Fujimura R, Azegami Y, Wei W, Kakuta H, Shiratori Y, Ohte N, et al. Distinct community composition of previously uncharacterized denitrifying bacteria and fungi across different land-use types. Microbes Environ. 2020;35:ME19064.
Scheu S, Ruess L, Bonkowski M. Interactions between microorganisms and soil micro-and mesofauna. Microorganisms in soils: roles in genesis and functions. Berlin, Germany: Springer; 2005. p. 253–75.
Coleman DC, Callaham MA, Crossley Jr DA. In: Fundamentals of soil ecology. 3rd edn. Burlington, USA: Academic Press; 2017. p. 106–21.
Whitford W, Sobhy H. Effects of repeated drought on soil microarthropod communities in the northern Chihuahuan Desert. Biol Fertil Soils. 1999;28:117–20.
Benckiser G. Fauna in soil ecosystems: recycling processes, nutrient fluxes, and agricultural production. New York, USE: CRC Press; 1997. p. 225–45.
Crossley D Jr, Mueller BR, Perdue JC. Biodiversity of microarthropods in agricultural soils: relations to processes. Agric Ecosyst Environ. 1992;40:37–46.
Behan-Pelletier VM. Oribatid mite biodiversity in agroecosystems: role for bioindication. Agric Ecosyst Environ. 1999;74:411–23.
Lindberg N, Engtsson JB, Persson T. Effects of experimental irrigation and drought on the composition and diversity of soil fauna in a coniferous stand. J Appl Ecol. 2002;39:924–36.
De Ruiter P, Moore J, Zwart K, Bouwman L, Hassink J, Bloem J, et al. Simulation of nitrogen mineralization in the below-ground food webs of two winter wheat fields. J Appl Ecol. 1993;30:95–106.
Thakur MP, van Groenigen JW, Kuiper I, De Deyn GB. Interactions between microbial-feeding and predatory soil fauna trigger N2O emissions. Soil Biol Biochem. 2014;70:256–62.
Porre RJ, van Groenigen JW, De Deyn GB, de Goede RG, Lubbers IM. Exploring the relationship between soil mesofauna, soil structure and N2O emissions. Soil Biol Biochem. 2016;96:55–64.
Johari K, Saman N, Song ST, Chin CS, Kong H, Mat H. Adsorption enhancement of elemental mercury by various surface modified coconut husk as eco-friendly low-cost adsorbents. Int Biodeterior Biodegradation 2016;109:45–52.
Cardoen D, Joshi P, Diels L, Sarma PM, Pant D. Agriculture biomass in India: part 2. Post-harvest losses, cost and environmental impacts. Resour Conserv Recycl. 2015;101:143–53.
Crowther TW, Boddy L, Jones TH. Functional and ecological consequences of saprotrophic fungus–grazer interactions. ISME J. 2012;6:1992–2001.
Chu H, Hosen Y, Yagi KNO. N2O, CH4 and CO2 fluxes in winter barley field of Japanese Andisol as affected by N fertilizer management. Soil Biol Biochem. 2007;39:330–9.
Wei W, Isobe K, Shiratori Y, Nishizawa T, Ohte N, Ise Y, et al. Development of PCR primers targeting fungal nirK to study fungal denitrification in the environment. Soil Biol Biochem. 2015;81:282–6.
Wei W, Isobe K, Nishizawa T, Zhu L, Shiratori Y, Ohte N, et al. Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J. 2015;9:1954–65.
Gao N, Shen W, Camargo E, Shiratori Y, Nishizawa T, Isobe K, et al. Nitrous oxide (N2O)-reducing denitrifier-inoculated organic fertilizer mitigates N2O emissions from agricultural soils. Biol Fertil Soils. 2017;53:885–98.
Shen W, Xue H, Gao N, Shiratori Y, Kamiya T, Fujiwara T, et al. Effects of copper on nitrous oxide (N2O) reduction in denitrifiers and N2O emissions from agricultural soils. Biol Fertil Soils. 2020;56:39–51.
Haarløv N. A new modification of the Tullgren apparatus. J Anim Ecol. 1947;16:115–21.
Krantz GW, Walter DE. A manual of acarology. 3rd edn. Lubbock, TX: Texas Tech University Press; 2009.
Liyanage MD, Jayasekara KS, Fernandopulle MN. Effects of application of coconut husk and coir dust on the yield of coconut. Cocos. 1993;9:15–22.
Romero GQ, Benson WW. Biotic interactions of mites, plants and leaf domatia. Curr Opin Plant Biol. 2005;8:436–40.
Grostal R, O’Dowd DJ. Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia. 1994;97:308–15.
Norton AP, English-Loeb G, Belden E. Host plant manipulation of natural enemies: leaf domatia protect beneficial mites from insect predators. Oecologia. 2001;126:535–42.
Norton AP, English-Loeb G, Gadoury D, Seem RC. Mycophagous mites and foliar pathogens: leaf domatia mediate tritrophic interactions in grapes. Ecology. 2000;81:490–9.
Behan VM, Hill S. Feeding habits and spore dispersal of oribatid mites in the North American arctic. Rev Ecol Biol Sol. 1978;15:497–516.
Seastedt T. The role of microarthropods in decomposition and mineralization processes. Annu Rev Entomol. 1984;29:25–46.
Singh BK, Trivedi P. Microbiome and the future for food and nutrient security. Microb Biotechnol. 2017;10:50–3.
Acknowledgements
This study was financially supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (15KT0024 and 20K21303), the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry (26037B and 27004C), and grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (research program on the development of innovative technology) (30012B). We thank W. Shen, N. Gao, N. Kaneko, M. Ishii, T. Kamiya, S. Otsuka, C. Hayakawa, R. Fujimura, Y. Ise, Z. Xu, C. Taylor, M. Maeda, and the technical staff members of Niigata Agricultural Research Institute for their help during this study.
Author information
Authors and Affiliations
Contributions
HS, YS, and KS conceived of the study. YS designed the first field experiment and interpreted the raw data. YS and SO performed the field setup, gas sampling, and corn yield determination in the first field experiment. HS extracted the mites from coconut husks and identified the mites. HS, KS, and YS proposed the hypothesis. HS, YS, and KS designed the second field experiment. YS, HS, KS, and SO performed the field setup, gas sampling, soil sampling, and mite extraction in the second field experiment. HS counted and identified the mites in the second field experiment. HS designed and performed all in vitro experiments and soil microcosm experiments. YM supported DNA extraction, qPCR, and the relative data analysis. HS performed the statistical analyses. HS wrote the paper with contributions from KI and KS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shen, H., Shiratori, Y., Ohta, S. et al. Mitigating N2O emissions from agricultural soils with fungivorous mites. ISME J 15, 2427–2439 (2021). https://doi.org/10.1038/s41396-021-00948-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41396-021-00948-4
This article is cited by
-
Phytoseiid mites benefited from organic fertilization by increasing the population of Tyrophagus mites in apple orchards
Experimental and Applied Acarology (2024)
-
An agroecological structure model of compost—soil—plant interactions for sustainable organic farming
ISME Communications (2023)
-
Mites reduce microbial N2O emissions
Nature Reviews Microbiology (2021)