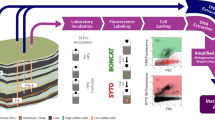
Abstract
Microbial metabolisms and interactions that facilitate subsurface conversions of recalcitrant carbon to methane are poorly understood. We deployed an in situ enrichment device in a subsurface coal seam in the Powder River Basin (PRB), USA, and used BONCAT-FACS-Metagenomics to identify translationally active populations involved in methane generation from a variety of coal-derived aromatic hydrocarbons. From the active fraction, high-quality metagenome-assembled genomes (MAGs) were recovered for the acetoclastic methanogen, Methanothrix paradoxum, and a novel member of the Chlorobi with the potential to generate acetate via the Pta-Ack pathway. Members of the Bacteroides and Geobacter also encoded Pta-Ack and together, all four populations had the putative ability to degrade ethylbenzene, phenylphosphate, phenylethanol, toluene, xylene, and phenol. Metabolic reconstructions, gene analyses, and environmental parameters also indicated that redox fluctuations likely promote facultative energy metabolisms in the coal seam. The active “Chlorobi PRB” MAG encoded enzymes for fermentation, nitrate reduction, and multiple oxygenases with varying binding affinities for oxygen. “M. paradoxum PRB” encoded an extradiol dioxygenase for aerobic phenylacetate degradation, which was also present in previously published Methanothrix genomes. These observations outline underlying processes for bio-methane from subbituminous coal by translationally active populations and demonstrate activity-based metagenomics as a powerful strategy in next generation physiology to understand ecologically relevant microbial populations.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Genomic sequence data associated with Total-sorted and BONCAT-sorted cells are available on the Integrated Microbial Genomes & Microbiomes (IMG) site under GOLD Study ID Gs014100. High quality MAGs for Methanothrix paradoxum PRB and Chlorobi PRB were submitted to JGI under GOLD Analysis IDs Ga0496496 and Ga0496497, respectively. Environmental metagenomes for FG11 and FGP wells are available on IMG under GOLD Project IDs Gp0406117 and Gp0406116, respectively.
References
Colosimo F, Thomas R, Lloyd JR, Taylor KG, Boothman C, Smith AD, et al. Biogenic methane in shale gas and coal bed methane: a review of current knowledge and gaps. Int J Coal Geol. 2016;165:106–20.
Strąpoć D, Mastalerz M, Dawson K, Macalady J, Callaghan AV, Wawrik B, et al. Biogeochemistry of microbial coal-bed methane. Annu Rev Earth Planet Sci. 2011;39:617–56.
Barnhart EP, Davis KJ, Varonka M, Orem W, Cunningham AB, Ramsay BD, et al. Enhanced coal-dependent methanogenesis coupled with algal biofuels: Potential water recycle and carbon capture. Int J Coal Geol. 2017;171:69–75.
Huang Z, Sednek C, Urynowicz MA, Guo H, Wang Q, Fallgren P, et al. Low carbon renewable natural gas production from coalbeds and implications for carbon capture and storage. Nat Commun. 2017;8:568.
Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2014. IPCC, Geneva, Switzerland.
Barnhart EP, Weeks EP, Jones EJP, Ritter DJ, McIntosh JC, Clark AC, et al. Hydrogeochemistry and coal-associated bacterial populations from a methanogenic coal bed. Int J Coal Geol. 2016;162:14–26.
Ritter D, Vinson D, Barnhart E, Akob DM, Fields MW, Cunningham AB, et al. Enhanced microbial coalbed methane generation: a review of research, commercial activity, and remaining challenges. Int J Coal Geol. 2015;146:28–41.
Zhuravlev YN, Porokhnov AN. Computer simulation of coal organic mass structure and its sorption properties. Int J Coal Sci Technol. 2019;6:438–44.
Sondreal EA, Wiltsee GA. Low-rank coal: its present and future role in the United States. Annu Rev Energy Environ. 1984;9:473–99.
Zhang R, Liu S, Bahadur J, Elsworth D, Wang Y, Hu G, et al. Changes in pore structure of coal caused by coal-to-gas bioconversion. Sci Rep. 2017;7:3840.
Lu Y, Chai C, Zhou Z, Ge Z, Yang M. Influence of bioconversion on pore structure of bituminous coal. Asia-Pac J Chem Eng. 2020;15:e2399.
Glombitza C, Mangelsdorf K, Horsfield B. A novel procedure to detect low molecular weight compounds released by alkaline ester cleavage from low maturity coals to assess its feedstock potential for deep microbial life. Org Geochem. 2009;40:175–83.
Jones EJP, Voytek MA, Corum MD, Orem WH. Stimulation of methane generation from nonproductive coal by addition of nutrients or a microbial consortium. Appl Environ Microbiol. 2010;76:7013–22.
Vinson DS, Blair NE, Ritter DJ, Martini AM, McIntosh JC. Carbon mass balance, isotopic tracers of biogenic methane, and the role of acetate in coal beds: Powder River Basin (USA). Chem Geol. 2019;530:119329.
Bapteste E, Brochier C, Boucher Y. Higher-level classification of the Archaea: evolution of methanogenesis and methanogens. Archaea. 2005;1:353–63.
Paul K, Nonoh JO, Mikulski L, Brune A. “Methanoplasmatales,” Thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of Methanogens. Appl Environ Microbiol. 2012;78:8245–53.
Vanwonterghem I, Evans PN, Parks DH, Jensen PD, Woodcroft BJ, Hugenholtz P, et al. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat Microbiol. 2016;1:16170.
Borrel G, Adam PS, McKay LJ, Chen L-X, Sierra-García IN, Sieber CMK, et al. Wide diversity of methane and short-chain alkane metabolisms in uncultured archaea. Nat Microbiol. 2019;4:603–13.
McKay LJ, Dlakić M, Fields MW, Delmont TO, Eren AM, Jay ZJ, et al. Co-occurring genomic capacity for anaerobic methane and dissimilatory sulfur metabolisms discovered in the Korarchaeota. Nat Microbiol. 2019;4:614–22.
Liu Y, Whitman WB. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann N. Y Acad Sci. 2008;1125:171–89.
Ferry JG. Acetate kinase and phosphotransacetylase. Methods Enzymol. 2011;494:219–31.
Jetten MSM, Stams AJM, Zehnder AJB. Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol Rev. 1992;8:181–97.
Zinder SH. Physiological Ecology of Methanogens. In: Ferry JG (ed). Methanogenesis: Ecology, Physiology, Biochemistry & Genetics. 1993. Springer US, Boston, MA, pp. 128–206.
Ferry JG. How to make a living by exhaling methane. Annu Rev Microbiol. 2010;64:453–73.
Kotsyurbenko OR, Chin K-J, Glagolev MV, Stubner S, Simankova MV, Nozhevnikova AN, et al. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ Microbiol. 2004;6:1159–73.
Conrad R. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol Ecol. 1999;28:193–202.
Schweitzer H, Ritter D, McIntosh J, Barnhart E, Cunningham AB, Vinson D, et al. Changes in microbial communities and associated water and gas geochemistry across a sulfate gradient in coal beds: Powder River Basin, USA. Geochim Cosmochim Acta. 2019;245:495–513.
Jetten MS, Stams AJ, Zehnder AJ. Isolation and characterization of acetyl-coenzyme A synthetase from Methanothrix soehngenii. J Bacteriol. 1989;171:5430–5.
Gujer W, Zehnder AJB. Conversion Processes in Anaerobic Digestion. Water Sci Technol; Lond. 1983;15:127–67.
Barnhart EP, Ruppert L, Hiebert R, Smith H, Schweitzer H, Clark A, et al. Injection of Deuterium and Yeast Extract at USGS Birney Field Site, Powder River Basin, Montana, USA, 2016–2020. US Geological Survey Data Release 2021.
Hatzenpichler R, Scheller S, Tavormina PL, Babin BM, Tirrell DA, Orphan VJ. In situ visualization of newly synthesized proteins in environmental microbes using amino acid tagging and click chemistry. Environ Microbiol. 2014;16:2568–90.
Hatzenpichler R, Connon SA, Goudeau D, Malmstrom RR, Woyke T, Orphan VJ. Visualizing in situ translational activity for identifying and sorting slow-growing archaeal- bacterial consortia. Proc Natl Acad Sci. 2016;113:E4069–78.
Couradeau E, Sasse J, Goudeau D, Nath N, Hazen TC, Bowen BP, et al. Probing the active fraction of soil microbiomes using BONCAT-FACS. Nat Commun. 2019;10:2770.
Reichart NJ, Jay ZJ, Krukenberg V, Parker AE, Spietz RL, Hatzenpichler R. Activity-based cell sorting reveals responses of uncultured archaea and bacteria to substrate amendment. ISME J. 2020;14:2851–61.
Schweitzer H, Smith H, Barnhart EP, McKay L, Gerlach R, Cunningham AB, et al. Subsurface Hydrocarbon Degradation Strategies in Low- and High-Sulfate Coal Seam Communities Identified with Activity-Based Metagenomics. bioRxiv 2021. https://doi.org/10.1101/2021.08.26.457739.
Angle JC, Morin TH, Solden LM, Narrowe AB, Smith GJ, Borton MA, et al. Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat Commun. 2017;8:1567.
Beckmann S, Luk AWS, Gutierrez-Zamora M-L, Chong NHH, Thomas T, Lee M, et al. Long-term succession in a coal seam microbiome during in situ biostimulation of coalbed-methane generation. ISME J. 2019;13:632–50.
Imhoff JF. Phylogenetic taxonomy of the family Chlorobiaceae on the basis of 16S rRNA and fmo (Fenna-Matthews-Olson protein) gene sequences. Int J Syst Evol Microbiol. 2003;53:941–51.
Iino T, Mori K, Uchino Y, Nakagawa T, Harayama S, Suzuki K-I. Ignavibacterium album gen. nov., sp. nov., a moderately thermophilic anaerobic bacterium isolated from microbial mats at a terrestrial hot spring and proposal of Ignavibacteria classis nov., for a novel lineage at the periphery of green sulfur bacteria. Int J Syst Evol Microbiol. 2010;60:1376–82.
Hugenholtz P, Pitulle C, Hershberger KL, Pace NR. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–76.
Hiras J, Wu Y-W, Eichorst SA, Simmons BA, Singer SW. Refining the phylum Chlorobi by resolving the phylogeny and metabolic potential of the representative of a deeply branching, uncultivated lineage. ISME J. 2016;10:833–45.
Evans PN, Parks DH, Chadwick GL, Robbins SJ, Orphan VJ, Golding SD, et al. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science. 2015;350:434–8.
Morris RL, Schmidt TM. Shallow breathing: bacterial life at low O(2). Nat Rev Microbiol. 2013;11:205–12.
Pitcher RS, Watmough NJ. The bacterial cytochrome cbb3 oxidases. Biochim Biophys Acta. 2004;1655:388–99.
Simon J, Pisa R, Stein T, Eichler R, Klimmek O, Gross R. The tetraheme cytochrome c NrfH is required to anchor the cytochrome c nitrite reductase (NrfA) in the membrane of Wolinella succinogenes. Eur J Biochem. 2001;268:5776–82.
Orellana LH, Rodriguez-R LM, Higgins S, Chee-Sanford JC, Sanford RA, Ritalahti KM, et al. Detecting nitrous oxide reductase (NosZ) genes in soil metagenomes: method development and implications for the nitrogen cycle. mBio. 2014;5:e01193–14.
Coates JD, Chakraborty R, Lack JG, O’Connor SM, Cole KA, Bender KS, et al. Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature. 2001;411:1039–43.
Chakraborty R, Coates JD. Anaerobic degradation of monoaromatic hydrocarbons. Appl Microbiol Biotechnol. 2004;64:437–46.
Muskotál A, Király R, Sebestyén A, Gugolya Z, Végh BM, Vonderviszt F. Interaction of FliS flagellar chaperone with flagellin. FEBS Lett. 2006;580:3916–20.
Nambu T, Kutsukake K. The Salmonella FlgA protein, a putativeve periplasmic chaperone essential for flagellar P ring formation. Microbiology. 2000;146:1171–8.
Liu Z, Frigaard N-U, Vogl K, Iino T, Ohkuma M, Overmann J, et al. Complete genome of ignavibacterium album, a metabolically versatile, flagellated, facultative anaerobe from the phylum chlorobi. Front Microbiol. 2012;3:185.
Ferry JG. Methane from acetate. J Bacteriol. 1992;174:5489–95.
Stams AJM, Teusink B, Sousa DZ. Ecophysiology of Acetoclastic Methanogens. In Stams AJM, Sousa DZ (eds.), Handbook of Hydrocarbon and Lipid Microbiology, Vol. 2 - Biogenesis of Hydrocarbons, Springer International Publishing AG, part of Springer Nature 2018, 2019. ISBN: 978-3-319-78107-5, 1–14.
Huser BA, Wuhrmann K, Zehnder AJB. Methanothrix soehngenii gen. nov. sp. nov., a new acetotrophic non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1982;132:1–9.
Callaghan AV, Wawrik B. AnHyDeg: a curated database of anaerobic hydrocarbon degradation genes. GitHub Oklahoma. 2016.
Mayumi D, Mochimaru H, Tamaki H, Yamamoto K, Yoshioka H, Suzuki Y, et al. Methane production from coal by a single methanogen. Science. 2016;354:222–5.
Botheju D, Samarakoon G, Chen C, Bakke R. An experimental study on the effects of oxygen in bio-gasification; Part 1. Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ 10). Granada, Spain: icrepq.com; 2010.
Angel R, Claus P, Conrad R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 2012;6:847–62.
Angel R, Matthies D, Conrad R. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen. PLoS One. 2011;6:e20453.
Krzycki JA, Zeikus JG. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984;158:231–7.
An D, Caffrey SM, Soh J, Agrawal A, Brown D, Budwill K, et al. Metagenomics of hydrocarbon resource environments indicates aerobic taxa and genes to be unexpectedly common. Environ Sci Technol. 2013;47:10708–17.
Barry KP, Taylor EA. Characterizing the promiscuity of LigAB, a lignin catabolite degrading extradiol dioxygenase from Sphingomonas paucimobilis SYK-6. Biochemistry. 2013;52:6724–36.
Galushko AS, Schink B. Oxidation of acetate through reactions of the citric acid cycle by Geobacter sulfurreducens in pure culture and in syntrophic coculture. Arch Microbiol. 2000;174:314–21.
Mahadevan R, Bond DR, Butler JE, Esteve-Nuñez A, Coppi MV, Palsson BO, et al. Characterization of metabolism in the Fe(III)-reducing organism Geobacter sulfurreducens by constraint-based modeling. Appl Environ Microbiol. 2006;72:1558–68.
Neuer G, Bothe H. The pyruvate: Ferredoxin oxidoreductase in heterocysts of the cyanobacteriuim Anabaena cylindrica. Biochimica et Biophysica Acta (BBA) - Gen Subj. 1982;716:358–65.
Furdui C, Ragsdale SW. The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J Biol Chem. 2000;275:28494–9.
Enjalbert B, Millard P, Dinclaux M, Portais J-C, Létisse F. Acetate fluxes in Escherichia coli are determined by the thermodynamic control of the Pta-AckA pathway. Sci Rep. 2017;7:42135.
Coskun ÖK, Pichler M, Vargas S, Gilder S, Orsi WD. Linking uncultivated microbial populations and benthic carbon turnover by using quantitative stable Isotope Probing. Appl Environ Microbiol. 2018;84:e01083–18.
Nolla-Ardèvol V, Peces M, Strous M, Tegetmeyer HE. Metagenome from a Spirulina digesting biogas reactor: analysis via binning of contigs and classification of short reads. BMC Microbiol. 2015;15:277.
Li Y, Liu M, Che X, Li C, Liang D, Zhou H, et al. Biochar stimulates growth of novel species capable of direct interspecies electron transfer in anaerobic digestion via ethanol-type fermentation. Environ Res. 2020;189:109983.
Li H-Y, Wang H, Wang H-T, Xin P-Y, Xu X-H, Ma Y, et al. The chemodiversity of paddy soil dissolved organic matter correlates with microbial community at continental scales. Microbiome. 2018;6:187.
Zeng Q, Huang L, Ma J, Zhu Z, He C, Shi Q, et al. Bio-reduction of ferrihydrite-montmorillonite-organic matter complexes: effect of montmorillonite and fate of organic matter. Geochim Cosmochim Acta. 2020;276:327–44.
Maier RM. Chapter 16 - Biogeochemical Cycling. In: Pepper IL, Gerba CP, Gentry TJ (eds). Environmental Microbiology (Third Edition). 2015. Academic Press, San Diego, pp. 339–73.
Stepanauskas R, Fergusson EA, Brown J, Poulton NJ, Tupper B, Labonté JM, et al. Improved genome recovery and integrated cell-size analyses of individual uncultured microbial cells and viral particles. Nat Commun. 2017;8:84.
Eren AM, Vineis JH, Morrison HG, Sogin ML. A filtering method to generate high quality short reads using illumina paired-end technology. PLoS One. 2013;8:e66643.
Li D, Liu C-M, Luo R, Sadakane K, Lam T-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–6.
Eren AM, Esen ÖC, Quince C, Vineis JH, Morrison HG, Sogin ML, et al. Anvi’o: an advanced analysis and visualization platform for ’omics data. PeerJ. 2015;3:e1319.
Pritchard L, Glover RH, Humphris S, Elphinstone JG, Toth IK. Genomics and taxonomy in diagnostics for food security: soft-rotting enterobacterial plant pathogens. Anal Methods. 2016;8:12–24.
Langmead B. Aligning short sequencing reads with Bowtie. Curr Protoc Bioinforma. 2010; Chapter 11: Unit 11.7.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Pruitt KD, Tatusova T, Maglott DR. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2005;33:D501–4.
Katoh K, Misawa K, Kuma K-I, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66.
Criscuolo A, Gribaldo S. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol. 2010;10:210.
Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinforma. 2010;11:119.
Kanehisa M, Goto S, Kawashima S, Nakaya A. The KEGG databases at GenomeNet. Nucleic Acids Res. 2002;30:42–6.
Zhou Z, Tran P, Liu Y, Kieft K, Anantharaman K. METABOLIC: A scalable high-throughput metabolic and biogeochemical functional trait profiler based on microbial genomes. Cold Spring Harbor Laboratory. 2019, 761643.
Duarte M, Jauregui R, Vilchez-Vargas R, Junca H, Pieper DH. AromaDeg, a novel database for phylogenomics of aerobic bacterial degradation of aromatics. Database. 2014;2014:bau118.
Molofsky LJ, Richardson SD, Gorody AW, Baldassare F, Black JA, McHugh TE, et al. Effect of different sampling methodologies on measured methane concentrations in groundwater samples. Ground Water. 2016;54:669–80.
Orem W, Tatu C, Varonka M, Lerch H, Bates A, Engle M, et al. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int J Coal Geol. 2014;126:20–31.
Acknowledgements
BONCAT-FACS and metagenomic sequencing were conducted under CSP503725 by the U.S. Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, which is supported under Contract No. DE-AC02-05CH11231. The authors (LJM, HJS, MWF) appreciate support from ENIGMA- Ecosystems and Networks Integrated with Genes and Molecular Assemblies (http://enigma.lbl.gov), a Science Focus Area Program at Lawrence Berkeley National Laboratory. We would like to thank Dr. Steven Singer at Lawrence Berkeley National Laboratory for sharing information regarding the Chlorobi NICIL-2 genome for comparison with Chlorobi PRB. We appreciate assistance in field work from Dr. Katie Davis and George Platt, and we are grateful to Dr. Jennifer MacIntosh and Dr. Daniel Ritter for geochemical analyses and discussion. We also acknowledge the USGS Energy Resources Program (Alicia Lindauer, Program Coordinator), the USGS National Innovation Center (Jonathan Stock, Director) and Montana Emergent Technologies for assistance in the field and SES development. Disclaimer: Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
HJS, HDS, EPB, LJM, and MWF designed the study. EPB, HJS, and HDS performed field sampling. HJS and HDS conducted laboratory experiments. RRM and DG performed cell sorting and sequencing. LJM, HDS, EPB, HJS, and MWF analyzed and interpreted the data. LJM, HJS, and EPB wrote the paper. All authors reviewed and edited the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
McKay, L.J., Smith, H.J., Barnhart, E.P. et al. Activity-based, genome-resolved metagenomics uncovers key populations and pathways involved in subsurface conversions of coal to methane. ISME J 16, 915–926 (2022). https://doi.org/10.1038/s41396-021-01139-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41396-021-01139-x
This article is cited by
-
An innovative strategy for overcoming ultra-high ammonia nitrogen inhibition on anaerobic methanogenesis via stepwise domestication
Microbiome (2025)
-
Metaproteomics and metagenomics reveal microbial pathways of organic matter degradation and methanogenesis in a marginally producing natural gas well
Communications Earth & Environment (2025)
-
Anthropogenic impacts on the terrestrial subsurface biosphere
Nature Reviews Microbiology (2025)
-
Recent progress on the biological degradation and solubilization of coal
Biodegradation (2025)
-
Metatranscriptomics-guided genome-scale metabolic reconstruction reveals the carbon flux and trophic interaction in methanogenic communities
Microbiome (2024)