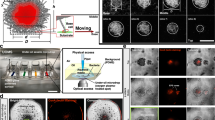
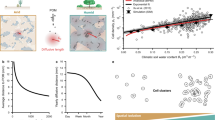
Abstract
Although migrations are essential for soil microorganisms to exploit scarce and heterogeneously distributed resources, bacterial mobility in soil remains poorly studied due to experimental limitations. In this study, time-lapse images collected using live microscopy techniques captured collective and coordinated groups of B. subtilis cells exhibiting “crowd movement”. Groups of B. subtilis cells moved through transparent soil (nafion polymer with particle size resembling sand) toward plant roots and re-arranged dynamically around root tips in the form of elongating and retracting “flocks” resembling collective behaviour usually associated with higher organisms (e.g., bird flocks or fish schools). Genetic analysis reveals B. subtilis flocks are likely driven by the diffusion of extracellular signalling molecules (e.g., chemotaxis, quorum sensing) and may be impacted by the physical obstacles and hydrodynamics encountered in the soil like environment. Our findings advance understanding of bacterial migration through soil matrices and expand known behaviours for coordinated bacterial movement.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
The link https://zenodo.org/record/4946262 provides the codes for numerical simulations of models of bacterial crowd movements. Processed image data and code for analysis of bacterial flocks is available at: https://github.com/LionelDupuy/CrowdMovement.
References
Kuzyakov Y, Razavi BS. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol Biochem. 2019;135:343–60.
Teixeira PJ, Colaianni NR, Fitzpatrick CR, Dangl JL. Beyond pathogens: Microbiota interactions with the plant immune system. Curr Opin Microbiol. 2019;49:7–17.
Alirezaeizanjani Z, Großmann R, Pfeifer V, Hintsche M, Beta C. Chemotaxis strategies of bacteria with multiple run modes. Sci Adv. 2020;6:eaaz6153.
Gao S, Wu H, Yu X, Qian L, Gao X. Swarming motility plays the major role in migration during tomato root colonization by Bacillus subtilis SWR01. Biol Control. 2016;98:11–17.
Mitchell JG, Kogure K. Bacterial Motility: Links to the environment and a driving force for microbial physics. FEMS Microbiol Ecol. 2006;55:3–16.
Kalamara M, Spacapan M, Mandic-Mulec I, Stanley-Wall NR. Social behaviours by Bacillus subtilis: Quorum sensing, kin discrimination and beyond. Mol Microbiol. 2018;110:863–78.
Posada LF, Álvarez JC, Romero-Tabarez M, de-Bashan L, Villegas-Escobar V. Enhanced molecular visualization of root colonization and growth promotion by Bacillus subtilis EA-CB0575 in different growth systems. Microbiol Res. 2018;217:69–80.
Beauregard PB, Yunrong C, Vlamakis H, Losick R, Kolter R. Bacillus subtilis Biofilm induction by plant polysaccharides. Proc Natl Acad Sci USA. 2013;110:1621–30.
Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier J. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 2016;7:1–10.
Massalha H, Korenblum E, Malitsky S, Shapiro OH, Aharoni A. Live imaging of root-bacteria interactions in a microfluidics setup. Proc Natl Acad Sci USA. 2017;114:4549–54.
Koch DL, Subramanian G. Collective hydrodynamics of swimming microorganisms: Living fluids. Annu Rev Fluid Mech. 2011;43:637–59.
Wioland H, Lushi E, Goldstein RE. Directed collective motion of bacteria under channel confinement. New J Phys. 2016;18:eaaz6153.
Petroff A, Libchaber A. Erratum: Hydrodynamics and collective behavior of the tethered bacterium Thiovulum majus. Proc Natl Acad Sci USA. 2016;111:5. E537-E545
Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–44.
Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004;134:307–19.
De Souza R, Ambrosini A, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015;38:401–19.
Roy K, Ghosh D, DeBruyn JM, Dasgupta T, Wommack KE, Liang X, et al. Temporal dynamics of soil virus and bacterial populations in agricultural and early plant successional soils. Front Microbiol. 2020;11:1–13.
Liu Y, Patko D, Engelhardt IC, George TS, Stanley-Wall NP, Ladmiral V. et al. Whole plant-environment microscopy reveals how Bacillus subtilis utilises the soil pore space to colonise plant roots. Proc Natl Acad Sci USA. 2021;118:e2109176118.
Einstein A. On the motion of small particles suspended in liquids at rest required by the molecular-kinetic theory of heat. Ann Phys. 1905;17:549–60.
Shellard A, Mayor R. Rules of Collective Migration: From the wildebeest to the neural crest: Rules of neural crest migration. Philos Trans R Soc B Biol Sci. 2020;375:1–9.
Torney CJ, Lamont M, Debell L, Angohiatok RJ, Leclerc LM, Berdahl AM. Inferring the rules of social interaction in migrating caribou. Philos Trans R Soc B Biol Sci. 2018;373:20170385.
Ballerini MN, Cabibbo R, Candelier A, Cavagna E, Cisbani I, Giardina V, et al. Interaction ruling animal collective behavior depends on topological rather than metric distance: Evidence from a field study. Proc Natl Acad Sci USA. 2008;105:1232–37.
Cavagna A, Cimarelli A, Giardina I, Parisi G, Santagati R, Stefanini F, et al. Scale-free correlations in starling flocks. Proc Natl Acad Sci USA. 2010;107:11865–70.
Katz Y, Tunstrøm C, Ioannou CC, Huepe C, Couzin ID. Inferring the structure and dynamics of interactions in schooling fish. Proc Natl Acad Sci USA. 2011;108:18720–25.
Buhl JD, Sumpter JT, Couzin ID, Hale JJ, Despland E, Miller ER, et al. From disorder to order in marching locusts. Science 2006;312:1402–6.
Seeley TD, Visscher PK. Quorum Sensing during nest-site selection by honeybee swarms. Behav Ecol Sociobiol. 2004;56:594–601.
Zhang HP, Be’er A, Florin EL, Swinney HL. Collective motion and density fluctuations in bacterial colonies. Proc Natl Acad Sci USA. 2010;107:13626–30.
Hughey LF, Hein AM, Strandburg-Peshkin A, Jensen FH. Challenges and solutions for studying collective animal behaviour in the wild. Philos Trans R Soc B Biol Sci. 2018;373:1–13.
Nadell CD, Xavier JB, Foster KR. The sociobiology of biofilms. FEMS Microbiol Rev. 2009;33:206–24.
Velicer GJ, Vos M. Sociobiology of the myxobacteria. Ann Rev Microbiol. 2009;63:599–623.
Branda SS, González-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA. 2001;98:11621–26.
Cordero OX, Wildschutte H, Kirkup B, Proehl S, Ngo L, Hussain F, et al. Antibiotic production and resistance. Sci Rep. 2012;337:1228–31.
Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. Myxobacteria: Moving, killing, feeding, and surviving together. Front Microbiol. 2016;7:1–18.
Li C, Hurley A, Hu W, Warrick JW, Lozano GL, Ayuso JM, et al. Social motility of biofilm-like microcolonies in a gliding bacterium. Nat Commun. 2021;12:1–12.
Sokolov A, Aranson IS, Kessler JO, Goldstein RE. Concentration dependence of the collective dynamics of swimming bacteria. Phys Rev Lett. 2007;98:158102.
Cisneros LH, Cortez R, Dombrowski C, Goldstein RE, Kessler JO. Fluid dynamics of self-propelled microorganisms, from individuals to concentrated populations. Exp Fluids. 2007;43:737–53.
Tuval I, Cisneros L, Dombrowski C, Wolgemuth CW, Kessler JO, Goldstein RE. Bacterial swimming and oxygen transport near contact lines. Proc Natl Acad Sci USA. 2005;102:2277–82.
Li G, Tam L, Tang JX. Amplified effect of brownian motion in bacterial near-surface swimming. Proc Natl Acad Sci USA. 2008;105:18355–59.
Lushi E, Wioland H, Goldstein RE. Fluid flows created by swimming bacteria drive self-organization in confined suspensions. Proc Natl Acad Sci USA. 2014;111:9733–38.
Ryan SD, Sokolov A, Berlyand L, Aranson IS. Correlation properties of collective motion in bacterial suspensions. New J Phys. 2013;15:105021.
Damton NC, Turner L, Rojevsky S, Berg HC. Dynamics of bacterial swarming. Biophys J. 2010;98:2082–90.
Ingham CJ, Jacob EB. Swarming and complex pattern formation in Paenibacillus vortex studied by imaging and tracking cells. BMC Microbiol. 2008;8:1–16.
Ariel G, Rabani A, Benisty S, Partridge JD, Harshey RM, Be’Er A. Swarming bacteria migrate by lévy walk. Nat Commun. 2015;6:8396.
Hamze K, Autret S, Hinc K, Laalami S, Julkowska D, Briandet R, et al. Single-cell analysis in situ in a Bacillus subtilis swarming community identifies distinct spatially separated subpopulations differentially expressing Hag (Flagellin), including specialized swarmers. Microbiol. 2011;157:2456–69.
Ghelardi E, Salvetti S, Ceragioli M, Gueye SA, Celandroni F, Senesi S. Contribution of surfactin and swrA to flagellin expression, swimming, and surface motility in Bacillus subtilis. Appl Environ Microbiol. 2012;78:6540–44.
Wilde A, Mullineaux CW. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol Rev. 2017;41:900–22.
Zhang J, Luo Y, Poh CL. Blue light-directed cell migration, aggregation, and patterning. J Mol Biol. 2020;432:3137–48.
Tian T, Sun B, Shi H, Gao T, He Y, Li Y, et al. Sucrose triggers a novel signalling cascade promoting Bacillus subtilis rhizosphere colonization. ISME J 2021;15:2723–37.
Harshey RM, Partridge JD. Shelter in a swarm. J Mol Biol. 2015;427:3683–94.
Burdett IDJ, Kirkwood TBL, Whalley JB. Growth kinetics of individual Bacillus subtilis cells and correlation with nucleoid extension. J Bacteriol. 1986;167:219–30.
Sharpe ME, Hauser PM, Sharpe RG, Errington J. Bacillus subtilis cell cycle as studied by fluorescence microscopy: Constancy of cell length at initiation of DNA replication and evidence for active nucleoid partitioning. J Bacteriol. 1998;180:547–55.
Rousk J, Bååth E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol Ecol. 2011;78:17–30.
Bennett RA, Lynch JM. Bacterial growth and development in the rhizosphere of gnotobiotic cereal plants. Microbiol. 1981;125:95–102.
Felici C, Vettori L, Giraldi E, Forino LMC, Toffanin A, Tagliasacchi AM, et al. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon Esculentum: Effects on plant growth and rhizosphere microbial community. Appl Soil Ecol. 2008;40:260–70.
Arkhipova TN, Galimsyanova NF, Kuzmina LY, Vysotskaya LB, Sidorova LV, Gabbasova IM, et al. Effect of seed bacterization with plant growth-promoting bacteria on wheat productivity and phosphorus mobility in the rhizosphere. Plant Soil Environ. 2019;65:313–19.
Marschner P, Crowley D, Rengel Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis - model and research methods. Soil Biol Biochem. 2011;43:883–94.
Lagos ML, Maruyama F, Nannipieri P, Mora ML, Jorquera MA. Current Overview on the study of bacteria in the rhizosphere by modern molecular techniques: A Mini-Review. J Soil Sci Plant Nutr. 2015;15:504–23.
Gerwig J, Kiley TB, Gunka K, Stanley-Wall N, Stülke J. The protein tyrosine kinases epsB and ptkA differentially affect biofilm formation in Bacillus Subtilis. Microbiol. 2014;160:682–91.
Shoesmith JG. The measurement of bacterial motility. J Gen Microbiol. 1960;22:528–35.
Schneider WR, Doetsch RN. Effect of viscosity on bacterial motility. J Bacteriol. 1974;117:696–701.
Kaiser GE, Doetsch RN. Enhanced translational motion of Leptospira in viscous environments. Nature 1975;255:656–57.
Ryan SD, Haines BM, Berlyand L, Ziebert F, Aranson IS. Viscosity of bacterial suspensions: Hydrodynamic interactions and self-induced noise. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;E83:050904.
López HM, Gachelin J, Douarche C, Auradou H, Clément E. Turning bacteria suspensions into superfluids. Phys Rev Lett. 2015;115:028301.
Butler MT, Wang Q, Harshey RM. Cell density and mobility protect swarming bacteria against antibiotics. Proc Natl Acad Sci USA. 2010;107:3776–81.
Erktan A, Or D, Scheu S. The physical structure of soil: Determinant and consequence of trophic interactions. Soil Biol Biochem. 2020;148:107876.
Rønn R, Thomsen IK, Jensen B. Naked amoebae, flagellates and nematodes in soil of different texture. Eur J Soil Biol. 1995;31:135–41.
Downie H, Holden N, Otten W, Spiers AJ, Valentine TA, Dupuy LX. Transparent soil for imaging the rhizosphere. PLoS ONE. 2012;7:1–6.
Mills AL. Keeping in Touch: Microbial life on soil particle surfaces. Adv Agron. 2003;78:1–43.
Downie HF, Valentine TA, Otten W, Spiers AJ, Dupuy LX. Transparent soil microcosms allow 3D spatial quantification of soil microbiological processes in vivo. Plant Signal Behav. 2014;9:e970421.
O’Callaghan FE, Braga RA, Neilson R, MacFarlane SA, Dupuy LX. New live screening of plant-nematode interactions in the rhizosphere. Sci Rep. 2018;8:1–17.
Sharma K, Palatinszky M, Nikolov G, Berry D, Shank EA. Transparent soil microcosms for live-cell imaging and non-destructive stable isotope probing of soil microorganisms. ELife 2020;9:1–28.
Bickel S, Or D. Soil bacterial diversity mediated by microscale aqueous-phase processes across biomes. Nat Commun. 2020;11:1–9.
Farré M, Sanchís J, Barceló D. Analysis and assessment of the occurrence, the fate and the behavior of nanomaterials in the environment. Trends Anal Chem. 2011;30:517–27.
Verhamme DT, Kiley TB, Stanley-Wall NR. DegU co-ordinates multicellular behaviour exhibited by Bacillus subtilis. Mol Microbiol. 2007;65:554–68.
Konkol MA, Blair KM, Kearns DB. Plasmid-encoded comi inhibits competence in the ancestral 3610 strain of Bacillus subtilis. J Bacteriol. 2013;195:4085–93.
Stanley NR, Lazazzera BA. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-γ-DL-glutamic acid production and biofilm formation. Mol Microbiol. 2005;57:1143–58.
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. URL https://www.R-project.org/.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.
Acknowledgements
The James Hutton Institute & Scotland’s Rural College were supported by funds from the Rural and Environment Science and Analytical Services Division of the Scottish Government. We thank Margarita Kalamara, Adam Ostrowski and the NSW laboratory for sharing their strains with us, Elliot Erskine, Jacqueline Marshall from JHI James Hutton Institute for advice on B. subtilis culture and other laboratory requirements, and Kathryn Wright from the James Hutton Institute for help with confocal microscopy. This work was funded by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement No. 647857-SENSOILS). We also acknowledge the funding from the Spanish Ministry of Science and Innovation (MICINN) under de project MICROCROWD (PID2020-112950RR-I00). Work in the NSW laboratory is funded by the Biotechnology and Biological Science Research Council (BBSRC) [BB/P001335/1, BB/R012415/1].
Author information
Authors and Affiliations
Contributions
Funding Acquisition: LD. Conceptualisation: LXD, TJD, NH, IE. Experimental Work: IE, DP, YL, GHM, TS. Data Analysis: IE, LD, MM. Mathematical Modelling: MM, MP, LXD. Supervision: TSG, MM, MP, NRS-W, NH, TJD, LXD. Validation: LE, MK, NSW, FAD. Visualisation: LE, MK. Writing – original draft: IE, LXD. Writing – review & editing: MM, TSG, MM, MP, NRS-W, NH, TJD, LXD.
Corresponding author
Ethics declarations
Competing interests
The material is original research, has not been previously published and has not been submitted for publication elsewhere while under consideration. The authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Engelhardt, I.C., Patko, D., Liu, Y. et al. Novel form of collective movement by soil bacteria. ISME J 16, 2337–2347 (2022). https://doi.org/10.1038/s41396-022-01277-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41396-022-01277-w
This article is cited by
-
Bacillus drives functional states in synthetic plant root bacterial communities
Genome Biology (2025)
-
Synthetic soil microcosms as emerging model systems to study the rhizosphere
Plant and Soil (2025)
-
Role of Bacillus subtilis exopolymeric genes in modulating rhizosphere microbiome assembly
Environmental Microbiome (2024)
-
Microbes in porous environments: from active interactions to emergent feedback
Biophysical Reviews (2024)
-
Characterization and environmental applications of soil biofilms: a review
Environmental Chemistry Letters (2024)