Abstract
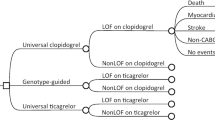
CYP2C19 loss of function (LOF) carriers undergoing percutaneous coronary intervention (PCI) have an increased risk of ischemic events when treated with clopidogrel. PCI patients in TAILOR-PCI were randomized to clopidogrel or genotype-guided (GG) therapy in which LOF carriers received ticagrelor and non-carriers clopidogrel. Direct medical costs associated with a GG approach have not been described before. TAILOR-PCI participants for whom direct medical costs were available for the duration from the date of PCI to one-year post PCI were included. Primary cost estimates were obtained from the Mayo Clinic Cost Data Warehouse. There were no differences in direct medical costs between the GG and clopidogrel groups (mean $20,682 versus $19,747, p = 0.11) however total costs were greater in the GG group (mean $21,245 versus $19,891, p = 0.02) which was primarily driven by ticagrelor costs. In conclusion the increased expense of a GG strategy post PCI as compared to clopidogrel for all is primarily driven by the cost of ticagrelor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Aggregate deidentified data could be made available based on participant consent to allow sharing of data from the corresponding author.
References
Mega JL, Simon T, Collet J, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: A meta-analysis. JAMA. 2010;304:1821–30.
Castrichini M, Luzum JA, Pereira N. Pharmacogenetics of antiplatelet therapy. Annu Rev Pharmacol Toxicol. 2023;63:211–29.
Brown SA, Pereira N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J Pers Med. 2018;8:8.
Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA. 2020;324:761–71.
Ingraham BS, Farkouh ME, Lennon RJ, So D, Goodman SG, Geller N, et al. Genetic-guided oral P2Y(12) inhibitor selection and cumulative ischemic events after percutaneous coronary intervention. JACC Cardiovasc Interv. 2023;16:816–25.
Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med. 2019;381:1621–31.
Parcha V, Heindl BF, Li P, Kalra R, Limdi NA, Pereira NL, et al. Genotype-guided P2Y(12) inhibitor therapy after percutaneous coronary intervention: A Bayesian analysis. Circ Genom Precis Med. 2021;14:e003353.
Visscher SL, Naessens JM, Yawn BP, Reinalda MS, Anderson SS, Borah BJ. Developing a standardized healthcare cost data warehouse. BMC Health Serv Res. 2017;17:396.
Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074–978.
Cavallari LH, Lee CR, Beitelshees AL, Cooper-DeHoff RM, Duarte JD, Voora D, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11:181–91.
Lee CR, Sriramoju VB, Cervantes A, Howell LA, Varunok N, Madan S, et al. Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med. 2018;11:e002069.
Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y(12) inhibitor therapy. a meta-analysis. JACC Cardiovasc Interv. 2021;14:739–50.
Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. 2021;397:1470–83.
Borah BJ, Pereira N, Farkouh ME. Personalizing antiplatelet therapies for acute coronary syndrome (ACS) in patients undergoing percutaneous coronary intervention (PCI): Are they cost-effective? Cardiovasc Drugs Ther. 2017;31:1–3.
Lala A, Berger JS, Sharma G, Hochman JS, Scott Braithwaite R, Ladapo JA. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost-effectiveness analysis. J Thromb Haemost. 2013;11:81–91.
Kim K, Touchette DR, Cavallari LH, Ardati AK, DiDomenico RJ. Cost-effectiveness of strategies to personalize the selection of P2Y(12) inhibitors in patients with acute coronary syndrome. Cardiovasc Drugs Ther. 2019;33:533–46.
Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J. 2022;43:959–67.
AlMukdad S, Elewa H, Al-Badriyeh D. Economic evaluations of CYP2C19 genotype-guided antiplatelet therapy compared to the universal use of antiplatelets in patients with acute coronary syndrome: A systematic review. J Cardiovasc Pharmacol Ther. 2020;25:201–11.
Claassens DMF, van Dorst PWM, Vos GJA, Bergmeijer TO, Hermanides RS, van ‘t Hof AWJ, et al. Cost effectiveness of a CYP2C19 genotype-guided strategy in patients with acute myocardial infarction: results from the POPular Genetics Trial. Am J Cardiovasc Drugs. 2022;22:195–206.
Dong OM, Friede KA, Chanfreau-Coffinier C, Voora D. Cost-effectiveness of CYP2C19-guided P2Y12 inhibitors in Veterans undergoing percutaneous coronary intervention for acute coronary syndromes. Eur Heart J Qual Care Clin Outcomes. 2023;9:249–57.
Funding
This work was supported by the NIH (grants U01HL128606 and U01HL128626).
Author information
Authors and Affiliations
Contributions
BB, NLP designed the study. SH, RL collected the data. AH, JM, RL, BB, NLP analyzed the data. AH, JM, MAH, RL, KB, BB, NLP interpreted the data. SH, JM, MAH, RL, KB, MB, NG, AL, VM, YR, MF, CR, BB, NLP prepared, reviewed, revised and provided final approval of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
BB is a consultant for Exact Sciences and Boehringer Ingelheim on projects unrelated to this project.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huxley, S., Moriarty, J., Hlatky, M.A. et al. Cost analysis of CYP2C19 genetic testing in percutaneous coronary intervention patients. Pharmacogenomics J 24, 32 (2024). https://doi.org/10.1038/s41397-024-00353-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00353-y
This article is cited by
-
CYP2C19 point-of-care testing: where are we now and where should we go?
The Pharmacogenomics Journal (2025)