Abstract
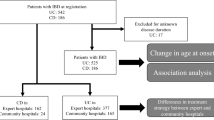
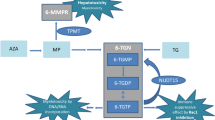
Thiopurine drugs are cornerstone treatment for patients with inflammatory bowel disease (IBD). The most common adverse drug reaction is thiopurine-induced myelosuppression (TIM), that may partly be explained by the genetic polymorphism NUDT15*3. The aim of this retrospective study was to determine the NUDT15*3 polymorphism frequency and its association with TIM in an IBD patient population in the Netherlands. DNA from patients previously genotyped for TPMT was genotyped for NUDT15*3. In IBD patients treated with thiopurines association tests with TIM were conducted. Out of 988 included patients, 13 (1.3%) were heterozygous for NUDT15*3. Of all patients, 606 had IBD and received thiopurine treatment. In these patients, 8/606 (1.3%) were heterozygous polymorphic for NUDT15*3 of which 50.0% developed TIM compared to 2.3% in the wild type patients (p < 0.001). The study results show a clinically relevant prevalence of NUDT15*3 in the Dutch patient population. Its strong association with TIM suggests pre-therapeutic genotyping potentially clinically utile.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Derijks LJJ, Gilissen LPL, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:715–29.
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485 LP–489.
Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–800.
Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, et al. Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis. 2017;23:1592–9.
Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–73.
Zhu X, Wang XD, Chao K, Zhi M, Zheng H, Ruan HL, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther. 2016;44:967–75.
Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2016;51:22–9.
Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–10.
Lee YJ, Hwang EH, Park JH, Shin JH, Kang B, Kim SY. NUDT15 variant is the most common variant associated with thiopurine-induced early leukopenia and alopecia in Korean pediatric patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2016;28:475–8.
Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–7.
Walker GJ, Harrison JW, Heap GA, Voskuil MD, Andersen V, Anderson CA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA J Am Med Assoc. 2019;321:753–61.
Moyer AM. NUDT15: a bench to bedside success story. Clin Biochem. 2021;92:1–8.
Zaza G, Meyling C, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. Thiopurine Pathway [Internet]. Pharmacogenetics and genomics. 2010 [cited 2022 Sep 23]. Available from: https://www.pharmgkb.org/pathway/PA2040
Matsuoka K. NUDT15 gene variants and thiopurine-induced leukopenia in patients with inflammatory bowel disease. Intest Res. 2020;18:275–81.
KNMP. Algemene achtergrondtekst Farmacogenetica - NUDT15 [Internet]. 2018 [cited 2022 Sep 19]. Available from: https://www.knmp.nl/downloads/g-standaard/farmacogenetica/achtergrondtekst-Farmacogenetica-CYP2D6-feb2020.pdf/view
Derijks LJJ, Wong DR, Hommes DW, van Bodegraven AA. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57:1075–106.
Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. 2018;53:1065–78.
Dean L. Azathioprine therapy and TPMT and NUDT15 genotype [Internet]. Med Genet Summaries. 2012. Available from: https://www.ncbi.nlm.nih.gov/books/
Tanaka Y, Saito Y. Importance of NUDT15 Polymorphisms in Thiopurine Treatments. J Pers Med. 2021;11:778.
Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017–20.
Yin D, Xia X, Zhang J, Zhang S, Liao F, Zhang G, et al. Impact of NUDT15 polymorphisms on thiopurines-induced myelotoxicity and thiopurines tolerance dose. Oncotarget. 2017;8:13575–85.
Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280–5.
Khaeso K, Udayachalerm S, Komvilaisak P, Chainansamit SO, Suwannaying K, Laoaroon N, et al. Meta-Analysis of NUDT15 Genetic Polymorphism on Thiopurine-Induced Myelosuppression in Asian Populations. Front Pharmacol. 2021;12:784712.
PHARMGKB. rs116855232 [Internet]. [cited 2023 Jan 19]. Available from: https://www.pharmgkb.org/variant/PA166154759/overview.
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91.
Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Blood/bone marrow [Internet]. 2006 [cited 2022 Sep 19]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33:1235–42.
Fan X, Yin D, Men R, Xu H, Yang L. NUDT15 polymorphism confer increased susceptibility to thiopurine-induced leukopenia in patients with autoimmune hepatitis and related cirrhosis. Front Pharmacol. 2019;10:1–8.
Liu Y, Meng Y, Wang L, Liu Z, Li J, Dong W. Associations between the NUDT15 R139C polymorphism and susceptibility to thiopurine-induced leukopenia in Asians: a meta-analysis. Onco Targets Ther. 2018;11:8309–17.
Zhang AL, Yang J, Wang H, Lu JL, Tang S, Zhang XJ. Association of NUDT15 c.415C>T allele and thiopurine-induced leukocytopenia in Asians: a systematic review and meta-analysis. Ir J Med Sci. 2018;187:145–53.
von Muhlenbrock C, Estay C, Covarrubias N, Miranda J, Venegas M. The c.415C>T polymorphism in NUDT15 is more frequent than the polymorphisms in TPMT in Chilean patients who use thiopurine drugs. Pharmacogenet Genomics. 2023;33:161–3.
Díaz-Villamarín X, Fernández-Varón E, Rojas Romero MC, Callejas-Rubio JL, Cabeza-Barrera J, Rodríguez-Nogales A, et al. Azathioprine dose tailoring based on pharmacogenetic information: insights of clinical implementation. Biomed Pharmacother. 2023;168:115706.
Schaeffeler E, Jaeger SU, Klumpp V, Yang JJ, Igel S, Hinze L, et al. Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet Med. 2019;21:2145–50.
Akiyama S, Matsuoka K, Fukuda K, Hamada S, Shimizu M, Nanki K, et al. Long-term effect of NUDT15 R139C on hematologic indices in inflammatory bowel disease patients treated with thiopurine. J Gastroenterol Hepatol. 2019;34:1751–7.
Yu N, Sriranganathan D, Walker GJ, Sazonovs A, Wilding H, Roberts C, et al. Prevalence of NUDT15 genetic variants and incidence of thiopurine-induced leukopenia in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2023;17:1920–30.
Han J, Xu J, Sun N, Jin S, Mei D, Wang X, et al. Analysis of mono-, di-, and triphosphates of thioguanosine and methylthioinosine in children with acute lymphoblastic leukemia by LC-MS/MS. J Pharm Biomed Anal. 2022;217:114813.
Vögelin M, Biedermann L, Frei P, Vavricka SR, Scharl S, Zeitz J, et al. The impact of azathioprine-associated lymphopenia on the onset of opportunistic infections in patients with inflammatory bowel disease. PLoS ONE. 2016;11:e0155218.
Funding
The study was sponsored by the Catharina Hospital.
Author information
Authors and Affiliations
Contributions
MJD: conceptualization, data curation, methodology, project administration, resources, supervision, writing—original draft. AJvN: data curation, methodology, formal analysis, investigation, visualization, writing—original draft. JBB: data curation, investigation, writing—review and editing. MAvD: data curation, resources, validation, writing—review and editing. JMS: resources, writing—review and editing. LJJD: conceptualization, methodology, writing—review and editing. LPLG: conceptualization, investigation, methodology, resources, validation, writing—review and editing. BALMD: conceptualization, data curation, funding acquisition, methodology, resources, writing—review and editing. All authors approved the final version of the manuscript, including the authorship list. MJD acts as the guarantor of the article and takes responsibility for the integrity of the work as a whole.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deenen, M.J., van Noordenburg, A.J., Bouwens-Bijsterveld, J. et al. Genetic association analysis and frequency of NUDT15*3 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease in a large Dutch cohort. Pharmacogenomics J 24, 39 (2024). https://doi.org/10.1038/s41397-024-00358-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41397-024-00358-7
This article is cited by
-
Population Pharmacokinetics Model of Thioguanine in Patients with Inflammatory Bowel Disease
Clinical Pharmacokinetics (2025)