Abstract
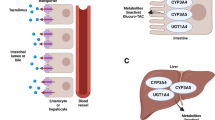
Liver transplantation is the only curative option for patients with advanced stages of liver disease, with tacrolimus used as the immunosuppressive drug of choice. Genetic variability can interfere with drug response, potentially leading to overexposure or underexposure. This study aims to investigate the association of CYP3A4 (rs2740574, rs2242480, rs35599367), CYP3A5 (rs776746, rs10264272), POR (rs1057868) and ABCB1 (rs1128503, rs2229109, rs9282564) gene polymorphisms with infection, acute rejection, and renal failure. The logistic regression model found an influence of CYP3A5 (rs776746) and POR28 (rs1057868) on the development of acute rejection after liver transplantation (p = 0.028). It also found an association between carriers of the variant allele of the POR*28 gene and infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Internal data management: After signing the informed consent form, clinical data was collected from the institution’s electronic medical records and databases and entered into the RedCAP platform (https://redcap.hc.fm.usp.br/). The polymorphism results were stored on the platform and on an institutional cloud drive (FMUSP). Only the researcher in charge and the master’s student have access to this data, which is protected by login and passwords. The data for statistical analysis with the other researchers was de-identified, thus ensuring the privacy, confidentiality and security of the participants.
References
Centers for Disease Control and Prevention: Chronic liver disease cirrhosis. 2020. https://www.cdc.gov/nchs/fastats/liver-disease.htm 2020.
Guimaraes J, Mesquita J, Kimura T, Oliveira A, Leite M, Oliveria A. Burden of liver diseases in Brazil, 1996-2022: epidemiology and impact to public healthcare. Lancet Reg Health Am. 2023;33:100731.
Adam R, Karam V, Cailliez V, O Grady JG, Mirza D, Cherqui D, et al. 2018 annual report of the european liver transplant registry (ELTR) – 50-year evolution of liver transplantation. Transpl Int. 2018;31:1293–317.
Bentata Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif Organs. 2019;44:140–52.
Di Maira T, Little EC, Berenguer M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol. 2020;46:101681.
European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64:433–85.
Jeng LB, Lee SG, Soin AS, Lee WC, Suh KS, Joo DJ, et al. Am J Transplant. 2018;18:1435–46.
Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl. 2012;18:1290–301.
Noble J, Terrec F, Malvezzi P, Rostaing L. Adverse effects of immunosuppression after liver transplantation. Best Pract Res Clin Gastroenterol. 2021;54-55:101762.
Rubín A, Sánchez-Montes C, Aguilera V, Juan FS, Ferrer I, Moya A, et al. Long-term outcome of “long-term liver transplant survivors”. Transpl Int. 2013;26:740–50.
Brunet M, Pastor-Anglada M. Insights into the pharmacogenetics of tacrolimus pharmacokinetics and pharmacodynamics. Pharmaceutics. 2022;14:1755.
Salvadori M, Tsalouchos A. Pharmacogenetics of immunosuppressant drugs: A new aspect for individualized therapy. World J Transplant. 2020;10:90–103.
Turolo S, Edefonti A, Syren ML, Montini G. Pharmacogenomics of old and new immunosuppressive drugs for precision medicine in kidney transplantation. J Clin Med. 2023;12:4454.
Rodriguez-Antona C, Savieo JL, Lauschke VM, Sangkuhl K, Drögemöller BI, Wang D, et al. PharmVar GeneFocus: CYP3A5. Clin Pharmacol Ther. 2022;112:1159–71.
Schutte-Nutgen K, Tholking G, Suwelack B, Reuter S. Tacrolimus – pharmacokinetic considerations for clinicians. Curr Drug Metab. 2018;19:342–50.
Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141–75.
Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98:19–24.
Hendijani F, Azarpira N, Kaviani M. Effect of CYP3A5*1 expression on tacrolimus required dose after liver transplantation: a systematic review and meta-analysis. Clin Transplant. 2018;32:e13306.
Uesugi M, Kikuchi M, Shinke H, Omura T, Yonezawa A, Matsubara K, et al. Impact of cytochrome P450 3A5 polymorphism in graft livers on the frequency of acute cellular rejection in living-donor liver transplantation. Pharmacogenet Genomics. 2014;24:356–66.
Shah RR. Pharmacogenetics in drug regulation: promise, potential and pitfalls. Philos Trans R Soc Lond B Biol Sci. 2005;360:1617–38.
Neuberger JM, Bechstein WO, Kuypers DRJ, Burra P, Citterio F, De Geest S, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation. 2017;101:S1–56.
Kelava T, Turcic P, Markotic A, Ostojic A, Sisl D, Mrzljak A. Importance of genetic polymorphisms in liver transplantation outcomes. World J Gastroenterol. 2020;26:1273–85.
Langman L, Van Gelder T, Van Schaik RHN Pharmacogenomics aspect of immunosuppressant therapy. Personalized Immunosuppression in Transplantation. 2016:109-24. https://doi.org/10.1016/B978-0-12-800885-0.00005-9.
Lee DH, Lee H, Yoon HY, Yee J, Gwak HS. Association of P450 oxidoreductase gene polymorphism with tacrolimus pharmacokinetics in renal transplant recipients: a systematic review and meta-analysis. Pharmaceutics. 2022;14:261.
Naslavsky M, Scliar M, Yamamoto G, Wang J, Zverinova S, Karp T, et al. Whole-genome sequencing of 1171 elderly admixed individuals from Brazil. Nature. 2022;13:1004.
Elens L, Hesselink DA, Bouamar R, Budde K, De Fijter JW, De Meyer M, et al. Impact of POR*28 on the pharmacokinetics of tacrolimus and cyclosporine a in renal transplant patients. 2014. http://www.drug-monitoring.com. Accessed 15 jun 2022.
Sridharan K, Shah S, Jassim A, Hammad M, Al Gadhban JE, Al Segai O. Evaluation of pharmacogenetics of drug-metabolizing enzymes and drug efflux transporter in renal transplants receiving immunosuppressants. J Pers Med. 2022;12:823.
Nakamura T, Fukuda M, Matsukane R, Suetsugu K, Harada N, Yoshizumi T, et al. Influence of POR*28 polymorphisms on 5*3-associated variations in tacrolimus blood levels at an early stage after liver transplantation. Int J Mol Sci. 2020;21:2287.
Gómez-Bravo MA, Salcedo M, Fondevila C, Suarez F, Castellote J, Rufian S, et al. Impact of donor and recipient CYP3A5 and ABCB1 genetic polymorphisms on tacrolimus dosage requirements and rejection in caucasian spanish liver transplant patients. J Clin Pharmacol. 2013;53:1146–54.
Woillard JB, Chouchana L, Picard N, Loriot MA. Pharmacogenetics of immunosuppressants: state of the art and clinical implementation – recommendations from the french national network of pharmacogenetics (RNPGx). Therapie. 2017;72:285–99.
Brunet M, Van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307.
U.S Food & Drug Administration. https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations. 2022.
Dong Y, Xu Q, Li R, Tao Y, Zhang Q, Li J, et al. CYP3A7, CYP3A4, and CYP3A5 genetic polymorphisms in recipients rather than donors influence tacrolimus concentrations in the early stages after liver transplantation. Gene. 2022;809:146007.
Maseko N, Yang S, Li C, Zhang S, Wang R, Zhang Y, et al. Impact of genetic polymorphisms on tacrolimus trough blood concentration in Chinese liver transplant recipients. Pharmacogenomics. 2023;24:207–17.
Anutrakulchai S, Pongskul C, Kritmetapak K, Limwattananon C, Vannaprasaht S. Therapeutic concentration achievement and allograft survival comparing usage of conventional tacrolimus doses and CYP3A5 genotype-guided doses in renal transplantation patients. Br J Clin Pharmacol. 2019;85:1964–73.
Katari SR, Magnone M, Shapiro R, Jordan M, Scantlebury V, Vivas C, et al. Clinical features of acute reversible tacrolimus (FK 506) nephrotoxicity in kidney transplant recipients. Clin Transplant. 1997;11:237–42.
Debette-Gratien M, Woillard JB, Picard N, Sebagh M, Loustaud-Ratti V, Sautereau D, et al. Influence of donor and recipient CYP3A4, CYP3A5, and ABCB1 genotypes on clinical outcomes and nephrotoxicity in liver transplant recipients. Transplantation. 2016;100:2129–37.
Kim V, Wal TV, Nishi MY, Montenegro LR, Carrilho FJ, Hoshida Y, et al. Brazilian cohort and genes encoding for drug-metabolizing enzymes and drug transporters. Pharmacogenomics. 2020;21:575–86.
Acknowledgements
We would like to thank FAPESP, CNPQ and CAPES for funding the project. We would also like to thank everyone involved in carrying out the project: nursing staff, medical staff, postgraduate secretary, laboratory team. Their participation was essential to the project’s progress. We would like to thank the Department of Gastroenterology, which provided the data of the patients on the waiting list for liver transplantation, collected and stored the blood of the liver transplant patients and helped collect the information of the liver donors. The HC-FMUSP Pharmacy Division also helped with access to data on immunosuppressant prescriptions for transplant patients.
Author information
Authors and Affiliations
Contributions
GDARN: data curation, formal analysis, writing – original draft, investigation, methodology, review & editing; ABM: supervision, visualization, writing – review & editing; TDMP: data curation, formal analysis, writing – review & editing; VF: data curation, formal analysis, review & editing; MAF: data curation, formal analysis, review & editing; GHG: data curation, review & editing; LN: data curation, clinical analysis, investigation, methodology, review & editing; LACDA: review and editing, supervision; RL: data curation, review & editing; EL: data curation, review & editing; ELRC: review and editing, supervision, resources; SKO: conceptualization, data curation, funding acquisition, methodology, project administration, supervision, validation, visualization, writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Financial support statement
FAPESP: 2019/00945-1; CNPq: 308609/2018-2; GDARN: CAPES Scholarship. The opinions, hypotheses, conclusions, or recommendations expressed in this material are the sole responsibility of the authors and do not necessarily reflect FAPESP’s, CNPq’s or CAPES’s view.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naldi, G.D.A.R., Minari, A.B., Pereira, T.D.M. et al. CYP3A5 and POR gene polymorphisms as predictors of infection and graft rejection in post-liver transplant patients treated with tacrolimus - a cohort study. Pharmacogenomics J 25, 4 (2025). https://doi.org/10.1038/s41397-025-00363-4
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41397-025-00363-4
This article is cited by
-
Impact of Pharmacogenomics on Transplant Outcomes: Review of Recent Literature
Current Transplantation Reports (2026)