Abstract
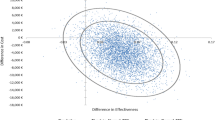
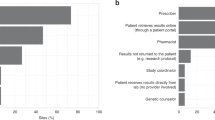
Tacrolimus (TAC) is one of the most widely prescribed maintenance immunosuppressant drugs in solid organ transplantation. Kidney transplantation is often the preferred treatment for patients with the kidney failure and is complemented with TAC treatment. TAC treatment is often associated with adverse drug events, which can be reduced by pharmacogenomic (PGx)-guided prescription. We conducted a cost-utility analysis to assess the cost-effectiveness of PGx-guided TAC treatment versus the conventional scheme in patients who recently underwent kidney transplantation in Slovenia. Clinical data were collected from the PREPARE study. The effectiveness of the treatment was determined by mean survival and utility values and Incremental Cost-Effectiveness Ratio was also calculated. Costs of PGx-guided treatment was comparable to conventional treatment but shared reduced risk for severe ADEs and 43% improved quality of life. PGx-guided arm showed a mean of 0.956 Quality-Adjusted Life Years (QALYs) (95% CI: 0.900–1.014) compared to 0.862 QALYs (95%CI: 0.801–0.918) in the other arm. Probabilistic sensitivity analyses confirmed the results’ robustness. In conclusion, PGx-guided treatment represents a cost-effective option for the TAC treatment of kidney-transplanted patients in the Slovenian healthcare setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
References
Hurwitz JT, Grizzle AJ, Tyler CS, Zapata LV, Malone DC. Cost-effectiveness of once-daily vs twice-daily tacrolimus among Hispanic and Black kidney transplant recipients. J Manag Care Spec Pharm. 2021;27:948–60.
Nobakht E, Jagadeesan M, Paul R, Bromberg J, Dadgar S. Precision medicine in kidney transplantation: just hype or a realistic hope? Transplant Direct. 2021;7:650–64.
Zaza G, Granata S, Tomei P, Dalla Gassa A, Lupo A. Personalization of the immunosuppressive treatment in renal transplant recipients: the great challenge in “omics” medicine. Int J Mol Sci. 2015;16:4281–305.
Arnol M, Naumovic R, Dimitrov EP, Racki S, Bucsa CA, Covic A, et al. Immunosuppressive regimens following kidney transplantation in five European countries: the observational RECORD study. Transplantation Reports. 2020;5:100061–672.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117–S314.
Thervet E, Loriot MA, Barbier S, Buchler M, Ficheux M, Choukroun G, et al. Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin Pharmacol Ther. 2010;87:721–6.
van Schaik RH, van der Heiden IP, van den Anker JN, Lindemans J. CYP3A5 variant allele frequencies in Dutch Caucasians. Clin Chem. 2002;48:1668–71.
Arnol M, Smrkolj T, Avsec D, Gadžijev A, Kneževič I. An increase in kidney transplantation procedures from deceased donors during the COVID-19 epidemic in Slovenia. Transpl Int. 2020;33:1562–4. https://doi.org/10.1111/tri.13715
Kandus A, Buturović Ponikvar J, Mlinšek G, Oblak M, Arnol M. Kidney Transplantation in Slovenia From 1970 to 2015. Ther Apher Dial. 2016;20:229–33.
Swen JJ, van der Wouden CH, Manson LE, Abdullah-Koolmees H, Blagec K, Blagus T, et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomized crossover implementation study. Lancet. 2023;401:347–56.
van der Wouden CH, Cambon-Thomsen A, Cecchin E, Cheung KC, Dávila-Fajardo CL, Deneer VH, et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther. 2017;101:341–58.
Gordois AL, Toth PP, Quek RG, Proudfoot EM, Paoli CJ, Gandra SR. Productivity losses associated with cardiovascular disease: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:759–69.
Willan AR, Briggs A Statistical analysis of cost‐effectiveness data. 1st ed., John Wiley & Sons Ltd, Canada, 2006.
Rancic N, Dragojevic-Simic V, Vavic N, Kovacevic A, Segrt Z, Djordjevic N. Economic evaluation of pharmacogenetic tests in patients subjected to renal transplantation: a review of literature. Front Public Health. 2016;4:189–97.
Chung R, Howard K, Craig JC, Chapman JR, Turner R, Wong G. Economic evaluations in kidney transplantation: frequency, characteristics, and quality-a systematic review. Transplantation. 2014;97:1027–33.
Fragoulakis V, Koufaki MI, Joefield-Roka C, Sunder-Plassmann G, Mitropoulou C. Cost-utility analysis of pharmacogenomics-guided tacrolimus treatment in Austrian kidney transplant recipients participating in the U-PGx PREPARE study. Pharmacogenomics J. 2024;24:10–16.
Morris SA, Alsaidi AT, Verbyla A, Cruz A, Macfarlane C, Bauer J, et al. Cost effectiveness of pharmacogenetic testing for drugs with Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines: a systematic review. Clin Pharmacol Ther. 2022;112:1318–28.
Verbelen M, Weale ME, Lewis CM. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17:395–402.
Stack AG, Yermak D, Roche DG, Ferguson JP, Elsayed M, Mohammed W, et al. Differential impact of smoking on mortality and kidney transplantation among adult Men and Women undergoing dialysis. BMC Nephrol. 2016;17:95–107.
Weinrauch LA, Claggett B, Liu J, Finn PV, Weir MR, Weiner DE, et al. Smoking and outcomes in kidney transplant recipients: a post hoc survival analysis of the FAVORIT trial. Int J Nephrol Renovasc Dis. 2018;11:155–64.
James A, Mannon RB. The cost of transplant immunosuppressant therapy: is this sustainable? Curr Transplant Rep. 2015;2:113–21.
Percy C, Hassoun Z, Mourad M, De Meyer M, Beguin C, Jadoul M, et al. Impact of acute infection requiring hospitalization on tacrolimus blood levels in kidney transplant recipients. Transplant Proc. 2017;49:2065–9.
Mizzi C, Dalabira E, Kumuthini J, Dzimiri N, Balogh I, Başak N, et al. A European spectrum of pharmacogenomic biomarkers: implications for clinical pharmacogenomics. PLoS One. 2016;11:e0162866.
Acknowledgements
This study has received funding from a European Commission grant (H2020-668353; U-PGx) to GPP, VD and CM.
Funding
The project was funded by the European Commission Horizon 2020 Program via Grant Agreement 668353 (U-PGx; www.upgx.eu).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception, design, and drafting of the paper. Data collection and documentation in eCRF were performed by the Slovenian clinical team (GM and JK) and researchers (TB and VD) working on the PREPARE study. CM, VD and GPP was one of the delegated persons, responsible for the design and the conduction of PREPARE project. VF and MIK reviewed and curated the study’s database. VF and MIK conducted data analysis. VF and MIK drafted the first version of the manuscript. CM and GPP drafted all subsequent versions of the manuscript. All authors reviewed the final version of the original and revised manuscript. VF and MIK are the guarantors for the overall content.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (0120-591/2016; KME 44/12/16).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fragoulakis, V., Koufaki, MI., Mlinšek, G. et al. Cost-utility analysis of pharmacogenomics-guided tacrolimus treatment of Slovenian patients undergoing kidney transplantation in the U-PGx PREPARE study. Pharmacogenomics J 25, 6 (2025). https://doi.org/10.1038/s41397-025-00365-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41397-025-00365-2