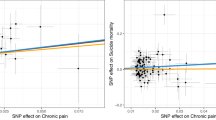
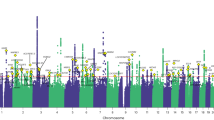
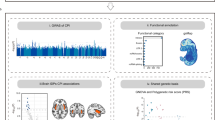
Abstract
Chronic pain represents heritable conditions linked to suicide death. It has been suggested that a shared genetic predisposition may contribute to this relationship, but there has not yet been a comprehensive assessment of genetic and clinical overlaps of different types of chronic pain with suicide death. Here, we integrated whole-genome sequencing and electronic health records from 986 unrelated individuals of European ancestry who died by suicide in the Utah Suicide Mortality Research Study and 415 ancestrally-matched population controls selected for absence of disease. Polygenic scores (PGSs) for seven distinct types of chronic pain were calculated and tested in the suicide cohort. We observed significant positive associations of PGSs for multisite chronic pain (PGSMCP) and chronic widespread pain (PGSCWP) with suicide mortality. Sex-stratified analyses showed elevations in both males and females. Pain diagnosis-stratified analyses revealed associations with suicide death regardless of chronic pain diagnoses. Follow-up tests of PGSs for more specific pain conditions showed additional associations with suicide death for: 1) monoarticular arthritis, 2) back pain, and 3) chronic inflammatory demyelinating polyneuropathy across all suicide death individuals, and 4) irritable bowel syndrome within males only. In a multiple logistic regression test of all chronic pain PGSs associating suicide death status, four types of pain remained uniquely associated with suicide death, highlighting distinct subgroups within suicide death: some attributed to MCP and CWP, and others associated with monoarticular arthritis or chronic inflammatory demyelinating polyneuropathy. This cohort study reports associations between suicide death and PGSs from various pain conditions, regardless of sex or chronic pain diagnosis, suggesting that combining genetic and clinical risk factors may better identify genetic overlap, causal directions, and/or specific gene pathways.
Similar content being viewed by others
Data availability
Publicly available GWAS datasets investigated in this study are available from the following sources. Multisite chronic pain: https://www.ebi.ac.uk/gwas/publications/31194737. Chronic widespread pain: https://zenodo.org/records/4459546. Monoarticular arthritis: https://www.ebi.ac.uk/gwas/publications/34737426. Back pain: https://zenodo.org/records/1319332, Chronic inflammatory demyelinating polyneuropathy: https://www.ebi.ac.uk/gwas/publications/34737426. Irritable bowel syndrome: https://www.ebi.ac.uk/gwas/publications/34741163, and Knee pain: https://www.ebi.ac.uk/gwas/publications/31482140. Additional data from this study is available from the authors upon request.
Code availability
PRSice-2: https://choishingwan.github.io/PRSice/; PRS-CS: https://github.com/getian107/PRScs; LDSC: https://github.com/bulik/ldsc; PLINK v1.9: https://www.cog-genomics.org/plink/1.9.
References
Kochanek KD, Xu J, Arias E Mortality in the United States, 2019. NCHS Data Brief 2020; 1-8.
Favril L, Yu R, Geddes JR, Fazel S. Individual-level risk factors for suicide mortality in the general population: an umbrella review. Lancet Public Health. 2023;8:e868–e877.
Stone DM, Simon TR, Fowler KA, Kegler SR, Yuan K, Holland KM, et al. Vital signs: trends in State Suicide Rates - United States, 1999-2016 and circumstances contributing to suicide - 27 States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:617–24.
Xiao Y, Bi K, Yip PS, Cerel J, Brown TT, Peng Y, et al. Decoding suicide decedent profiles and signs of suicidal intent using latent class analysis. JAMA Psychiatry. 2024;81:595–605.
Coon H, Shabalin AA, DiBlasi E, Monson ET, Han S, Kaufman EA, et al. Absence of nonfatal suicidal behavior preceding suicide death reveals differences in clinical risks. Psychiatry Res. 2025;347:116391.
Ilgen MA, Kleinberg F, Ignacio RV, Bohnert AS, Valenstein M, McCarthy JF, et al. Noncancer pain conditions and risk of suicide. JAMA Psychiatry. 2013;70:692–7.
Chincholkar M, Blackshaw S. Suicidality in chronic pain: assessment and management. BJA Educ. 2023;23:320–6.
Adawi M, Chen W, Bragazzi NL, Watad A, McGonagle D, Yavne Y, et al. Suicidal behavior in fibromyalgia patients: rates and determinants of suicide ideation, risk, suicide, and suicidal attempts-a systematic review of the literature and meta-analysis of over 390,000 fibromyalgia patients. Front Psychiatry. 2021;12:629417.
Eckhoff C, Straume B, Kvernmo S. Multisite musculoskeletal pain in adolescence and later mental health disorders: a population-based registry study of Norwegian youth: the NAAHS cohort study. BMJ Open. 2017;7:e012035.
Ryan PC, Lowry NJ, Boudreaux E, Snyder DJ, Claassen CA, Harrington CJ. et al. Chronic pain, hopelessness, and suicide risk among adult medical inpatients. J Acad Consult Liaison Psychiatry. 2024;65:126–35.
Hinze V, Karl A, Ford T, Gjelsvik B. Pain and suicidality in children and adolescents: a longitudinal population-based study. Eur Child Adolesc Psychiatry. 2023;32:1507–17.
Bhatt RR, Haddad E, Zhu AH, Thompson PM, Gupta A, Mayer EA, et al. Mapping brain structure variability in chronic pain: the role of widespreadness and pain type and its mediating relationship with suicide attempt. Biol Psychiatry. 2023;95:473–481.
Ilgen MA, Zivin K, McCammon RJ, Valenstein M. Pain and suicidal thoughts, plans and attempts in the United States. Gen Hosp Psychiatry. 2008;30:521–7.
DiBlasi E, Kaufman EA, Webster S, Hagn EE, Shabalin AA, Chen D, et al. Phenome-wide diagnostic comparison among suicide deaths and living individuals with chronic pain diagnoses. BMC Med. 2024;22:568.
Freeman A, Mergl R, Kohls E, Szekely A, Gusmao R, Arensman E, et al. A cross-national study on gender differences in suicide intent. BMC Psychiatry. 2017;17:234.
Padron-Monedero A, Noguer-Zambano I, Arleth AG, Martin MP, Ruiz JS. Sex differences in death by suicide versus the rest of external causes of death across the lifespan. a population study. Eur J Psychiatry. 2025;39:100261.
Smith AF, Plumb AN, Berardi G, Sluka KA. Sex differences in the transition to chronic pain. J Clin Invest. 2025;135:e191931.
Kuan AS, Wang YF, Chen SP, Chuang YF, Wang SJ. Sex differences in pain, suicidal ideation, and suicide attempts in patients with migraine. Headache. 2025;65:983–93.
Hocking LJ, Generation S, Morris AD, Dominiczak AF, Porteous DJ, Smith BH. Heritability of chronic pain in 2195 extended families. Eur J Pain. 2012;16:1053–63.
Quintero Reis A, Newton BA, Kessler R, Polimanti R, Wendt FR. Functional and molecular characterization of suicidality factors using phenotypic and genome-wide data. Mol Psychiatry. 2023;28:1064–71.
Chen C, Pettersson E, Summit AG, Boersma K, Chang Z, Kuja-Halkola R, et al. Chronic pain conditions and risk of suicidal behavior: a 10-year longitudinal co-twin control study. BMC Med. 2023;21:9.
Fiore NT, Debs SR, Hayes JP, Duffy SS, Moalem-Taylor G. Pain-resolving immune mechanisms in neuropathic pain. Nat Rev Neurol. 2023;19:199–220.
Tang Y, Liu W, Kong W, Zhang S, Zhu T. Multisite chronic pain and the risk of autoimmune diseases: A Mendelian randomization study. Front Immunol. 2023;14:1077088.
Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1–27.
Ikeda M, Saito T, Kanazawa T, Iwata N. Polygenic risk score as clinical utility in psychiatry: a clinical viewpoint. J Hum Genet. 2021;66:53–60.
Huang Y, Chen D, Levin AM, Ahmedani BK, Frank C, Li M, et al. Cross-phenotype relationship between opioid use disorder and suicide attempts: new evidence from polygenic association and Mendelian randomization analyses. Mol Psychiatry. 2023;28:2913–21.
Wootton O, Shadrin AA, Mohn C, Susser E, Ramesar R, Gur RC, et al. Genome-wide association study in 404,302 individuals identifies 7 significant loci for reaction time variability. Mol Psychiatry. 2023;28:4011–9.
Monson ET, Shabalin AA, Docherty AR, DiBlasi E, Bakian AV, Li QS, et al. Assessment of suicide attempt and death in bipolar affective disorder: a combined clinical and genetic approach. Transl Psychiatry. 2021;11:379.
Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018;19:581–90.
Sun J, Yan W, Zhang XN, Lin X, Li H, Gong YM, et al. Polygenic evidence and overlapped brain functional connectivities for the association between chronic pain and sleep disturbance. Transl Psychiatry. 2020;10:252.
Li QS, Shabalin AA, DiBlasi E, Gopal S, Canuso CM, Finn GenISGC, et al. Genome-wide association study meta-analysis of suicide death and suicidal behavior. Mol Psychiatry. 2023;28:891–900.
Docherty AR, Mullins N, Ashley-Koch AE, Qin X, Coleman JRI, Shabalin A, et al. GWAS Meta-Analysis of suicide attempt: identification of 12 genome-wide significant loci and implication of genetic risks for specific health factors. Am J Psychiatry. 2023;180:723–38.
Docherty AR, Shabalin AA, DiBlasi E, Monson E, Mullins N, Adkins DE, et al. Genome-Wide association study of suicide death and polygenic prediction of clinical antecedents. Am J Psychiatry. 2020;177:917–27.
Coon H, Shabalin A, Bakian AV, DiBlasi E, Monson ET, Kirby A, et al. Extended familial risk of suicide death is associated with younger age at death and elevated polygenic risk of suicide. Am J Med Genet B Neuropsychiatr Genet. 2022;189:60–73.
Han S, DiBlasi E, Monson ET, Shabalin A, Ferris E, Chen D. et al. Whole-genome sequencing analysis of suicide deaths integrating brain-regulatory eQTLs data to identify risk loci and genes. Mol Psychiatry. 2023;28:3909–19.
Bastarache L. Using phecodes for research with the electronic health record: from PheWAS to PheRS. Annu Rev Biomed Data Sci. 2021;4:1–19.
Denny JC, Bastarache L, Ritchie MD, Carroll RJ, Zink R, Mosley JD, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol. 2013;31:1102–10.
Byrska-Bishop M, Evani US, Zhao X, Basile AO, Abel HJ, Regier AA, et al. High-coverage whole-genome sequencing of the expanded 1000 genomes project cohort including 602 trios. Cell. 2022;185:3426–3440.e3419.
Tschanz JT, Corcoran C, Skoog I, Khachaturian AS, Herrick J, Hayden KM, et al. Dementia: the leading predictor of death in a defined elderly population: the cache county study. Neurology. 2004;62:1156–62.
Dausset J, Cann H, Cohen D, Lathrop M, Lalouel JM, White R. Centre d’etude du polymorphisme humain (CEPH): collaborative genetic mapping of the human genome. Genomics. 1990;6:575–7.
Johnston KJA, Adams MJ, Nicholl BI, Ward J, Strawbridge RJ, Ferguson A, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15:e1008164.
Rahman MS, Winsvold BS, Chavez Chavez SO, Borte S, Tsepilov YA, Sharapov SZ, et al. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann Rheum Dis. 2021;80:1227–35.
liftOver: changing genomic coordinate systems with Rtracklayer. R package version 1.28.0.
Choi SW, O’Reilly PF. PRSice-2: polygenic risk score software for biobank-scale data. Gigascience. 2019;8:giz082.
Zhou G, Zhao H. A fast and robust bayesian nonparametric method for prediction of complex traits using summary statistics. PLoS Genet. 2021;17:e1009697.
Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth’s logistic regression with rare events: accurate effect estimates and predictions?. Stat Med. 2017;36:2302–17.
Patel AP, Wang M, Ruan Y, Koyama S, Clarke SL, Yang X, et al. A multi-ancestry polygenic risk score improves risk prediction for coronary artery disease. Nat Med. 2023;29:1793–803.
Dennis J, Sealock J, Levinson RT, Farber-Eger E, Franco J, Fong S, et al. Genetic risk for major depressive disorder and loneliness in sex-specific associations with coronary artery disease. Mol Psychiatry. 2021;26:4254–64.
Yoon N, Cho YS. Development of a polygenic risk score for BMI to assess the genetic susceptibility to obesity and related diseases in the Korean population. Int J Mol Sci. 2023;24:11560.
Choe EK, Shivakumar M, Lee SM, Verma A, Kim D. Dissecting the clinical relevance of polygenic risk score for obesity-a cross-sectional, longitudinal analysis. Int J Obes. 2022;46:1686–93.
Nurnberger JI Jr., Wang Y, Zang Y, Lai D, Wetherill L, Edenberg HJ, et al. High polygenic risk scores are associated with early age of onset of alcohol use disorder in adolescents and young adults at risk. Biol Psychiatry Glob Open Sci. 2022;2:379–88.
Collister JA, Liu X, Clifton L. Calculating polygenic risk scores (PRS) in UK biobank: a practical guide for epidemiologists. Front Genet. 2022;13:818574.
Cupido AJ, Tromp TR, Hovingh GK. The clinical applicability of polygenic risk scores for LDL-cholesterol: considerations, current evidence and future perspectives. Curr Opin Lipidol. 2021;32:112–6.
Kamburov A, Herwig R. ConsensusPathDB 2022: molecular interactions update as a resource for network biology. Nucleic Acids Res. 2022;50:D587–D595.
Choi SW, Garcia-Gonzalez J, Ruan Y, Wu HM, Porras C, Johnson J, et al. PRSet: pathway-based polygenic risk score analyses and software. PLoS Genet. 2023;19:e1010624.
Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. 2021;53:1616–21.
Freidin MB, Tsepilov YA, Palmer M, Karssen LC, Suri P, Aulchenko YS, et al. Insight into the genetic architecture of back pain and its risk factors from a study of 509,000 individuals. Pain. 2019;160:1361–73.
Eijsbouts C, Zheng T, Kennedy NA, Bonfiglio F, Anderson CA, Moutsianas L, et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet. 2021;53:1543–52.
Meng W, Adams MJ, Palmer CNA, andMe Research T, Shi J, Auton A, et al. Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK biobank. Commun Biol. 2019;2:321.
Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46:1734–9.
Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512–25.
Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–8.
Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–8.
Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2.
Owen-Smith AA, Ahmedani BK, Peterson E, Simon GE, Rossom RC, Lynch FL, et al. The mediating effect of sleep disturbance on the relationship between nonmalignant chronic pain and suicide death. Pain Pract. 2019;19:382–9.
Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med. 2001;134:868–81.
Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping Chronic Pain Conditions: Implications for Diagnosis and Classification. J Pain. 2016;17:T93–T107.
Kamaleri Y, Natvig B, Ihlebaek CM, Benth JS, Bruusgaard D. Number of pain sites is associated with demographic, lifestyle, and health-related factors in the general population. Eur J Pain. 2008;12:742–8.
Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states-maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–54.
Nicholl BI, Mackay D, Cullen B, Martin DJ, Ul-Haq Z, Mair FS, et al. Chronic multisite pain in major depression and bipolar disorder: cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry. 2014;14:350.
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383.
Michaelides A, Hadden RDM, Sarrigiannis PG, Hadjivassiliou M, Zis P. Pain in chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta-analysis. Pain Ther. 2019;8:177–85.
Erlangsen A, Stenager E, Conwell Y, Andersen PK, Hawton K, Benros ME, et al. Association between neurological disorders and death by suicide in Denmark. JAMA. 2020;323:444–54.
Damci A, Schruers KRJ, Leue C, Faber CG, Hoeijmakers JGJ. Anxiety and depression in small fiber neuropathy. J Peripher Nerv Syst. 2022;27:291–301.
Radat F, Margot-Duclot A, Attal N. Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain. 2013;17:1547–57.
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32.
Lehmann HC, Meyer Zu Horste G, Kieseier BC, Hartung HP. Pathogenesis and treatment of immune-mediated neuropathies. Ther Adv Neurol Disord. 2009;2:261–81.
Balit J, Erlangsen A, Docherty A, Turecki G, Orri M. Association of chronic pain with suicide attempt and death by suicide: a two-sample Mendelian randomization. Mol Psychiatry. 2024;29:2043–9.
Funding
This work was supported by the National Institutes of Health (R01MH122412, R01MH123489, R01MH123619, R01ES032028); the American Foundation for Suicide Prevention (VW, HC: BSG-1-005-18), the Brain & Behavior Research Foundation-NARSAD (ED, grant number 28132; AS grant number 28686; EM grant number 31248); and the Clark Tanner Foundation (HC, AS, EM, AB). Cellular Translational Research Core Services at the University of Utah are supported by NIH CTSA UM1 TR004409. Partial support for all datasets housed within the Utah Population Data Base is provided by the Huntsman Cancer Institute (HCI), http://www.huntsmancancer.org/, and the HCI Cancer Center Support grant, P30CA42014 from the National Cancer Institute. Whole-genome sequencing of suicide deaths was supported in part by a donation from the Huntsman Mental Health Institute. Research was supported by NCRR grant “Sharing statewide health data for genetic research” R01RR021746 with additional support from the Utah Department of Health and Human Services and the University of Utah. We thank the University of Utah Pedigree and Population Resource and the University of Utah Health Enterprise Data Warehouse for establishing the Master Subject Index between the Utah Population Database and the University of Utah Health Sciences Center. We additionally thank our colleagues at Intermountain Health for working with Utah Population Database staff in linkage and subsequent de-identification of IH health records data.
Author information
Authors and Affiliations
Contributions
SH, AD, and HC conceptualized and designed this study. SH, ED, EM, AS, DC, KE, AB, BK, HC, and AD contributed to analysis and interpretation of EHR and genomics data. LB, DC, DL, DT, EF were involved in data preparation. QL, VW, HC, and AD generated whole-genome sequencing data. All of the funding and work needed to generate whole-genome sequencing data includes only QL, FW, and HC. ZY and DC contributed to integration of clinical and demographic data with de-identification. WC, MS, and HC collected biosamples for suicide death. ED and AO were involved in defining suicide cases with chronic pain conditions and interpreting results in the context of chronic pain. SH, ED, EM, HC, and AD prepared the first draft of the manuscript. HC and AD supervised this study. All authors contributed to completing the manuscript by reading and revising it. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Qingqin S. Li is an employee of Janssen Research and Development. All other authors declare no conflicts of interest.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Ethical approval for this study is received annually from Institutional Review Boards of the University of Utah, Intermountain Health, and the Utah Department of Health and Human Services, and informed consent was obtained from all subjects prior to study participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, S., DiBlasi, E., Monson, E.T. et al. Genetic risk of chronic pain conditions associated with risk of suicide death through an integrative analysis of EHR and genomics data. Transl Psychiatry (2026). https://doi.org/10.1038/s41398-026-03861-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-026-03861-6