Abstract
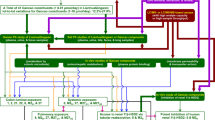
The influence of broad-spectrum antibiotics on the pharmacokinetics and biotransformation of major constituents of Shaoyao-Gancao decoction (SGD) in rats was investigated. The pharmacokinetic behaviors of paeoniflorin (PF), albiflorin (AF), liquiritin (LT), isoliquiritin (ILT), liquiritin apioside (LA), isoliquiritin apioside (ILA), and glycyrrhizic acid (GL), seven major constituents of SGD, as well as glycyrrhetinic acid (GA), a major metabolite of GL, were analyzed. A 1-week pretreatment with broad-spectrum antibiotics (ampicillin, metronidazole, neomycin, 1 g L−1; and vancomycin, 0.5 g L−1) via drinking water reduced plasma exposure of the major constituents. The AUC0-24 h of PF and LT was significantly decreased by 28.7% and 33.8% (P < 0.05 and P < 0.005), respectively. Although the differences were not statistically significant, the AUC0-24 h of AF, ILT, LA, ILA, and GL was decreased by 31.4%, 50.9%, 16.9%, 44.1%, and 37.0%, respectively, compared with the control group. In addition, the plasma GA exposure in the antibiotic-pretreated group was significantly lower (P < 0.005) than the control group. The in vitro stability of the major constituents of SGD in the rat intestinal contents with or without broad-spectrum antibiotics was also investigated. The major constituents were comparatively stable in the rat duodenum contents, and the biotransformation of GL mainly occurred in the rat colon contents. In summary, broad-spectrum antibiotics suppressed the absorption of the major constituents of SGD and significantly inhibited the biotransformation of GL to GA by suppressing the colon microbiota. The results indicated a potential clinical drug–drug interaction (DDI) when SGD was administered with broad-spectrum antibiotics.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ning YH, Guo CW. Analysis on the Clinical Research of Shaoyao Gancao Decoction according to 21 Articles. Guiding J Tradit Chin Med Pharm. 2017;23:83–4.
Katsura T. The remarkable effect of kanzo-to and shakuyakukanzo-to, in the treatment of acute abdominal pain. Jpn J Orient Med. 2010;46:293–9.
Hinoshita F, Ogura Y, Suzuki Y, Hara S, Yamada A, Tanaka N, et al. Effect of orally administered Shao-Yao-Gan-Cao-tang (Sha-kuyaku-kanzo-to) on muscle cramps in maintenance hemodialysis patients: a preliminary study. Am J Chin Med. 2003;31:445–53.
Zhu GW, Zhang GJ, Wang M, Wang JJ, Qu L, Cai YP. Research situation about origin, active components alignment and pharmacological actions of Shaoyao Gancao Decoction. China J Tradit Chin Med Pharm. 2015;30:2865–9.
Sui F, Zhou HY, Meng J, Du XL, Sui YP, Zhou ZK, et al. A Chinese herbal decoction, Shaoyao-Gancao Tang, exerts analgesic effect by down-regulating the TRPV1 channel in a rat model ofarthritic pain. Am J Chin Med. 2016;44:1363–78.
Zhang Y, Jia XL, Yang J, Li Q, Yan GF, Xu ZJ, et al. Effects of Shaoyao-Gancao decoction on infarcted cerebral cortical neurons: suppression of the inflammatory response following cerebral ischemia-reperfusion in a rat model. Biomed Res Int. 2016;2016:1859252.
Cheng C, Lin JZ, Li L, Yang JL, Jia WW, Huang YH, et al. Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol Sin. 2016;37:530–44.
Zhang QY, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A. 2009;1216:1954–69.
Wang P, Yin QW, Zhang AH, Sun H, Wu XH, Wang XJ. Preliminary identification of the absorbed bioactive components and metabolites in rat plasma after oral administration of Shaoyao-Gancao decoction by ultra-performance liquid chromatography with electrospray ioni-zation tandem mass spectrometry. Pharmacogn Mag. 2014;10:497–502.
Peng DC, Wang HS, Qu CL, Xie LH, Wicks SM, Xie JT. Ginsenoside Re: its chemistry, metabolism and pharmacokinetics. Chin Med. 2012;7:2.
Zhou W, Di LQ, Wang J, Shan JJ, Liu SJ, Ju WZ, et al. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol Sin. 2012;33:1069–79.
Liu JY, Lee KF, Sze CW, Tong Y, Tang SCW, Ng TB, et al. Intestinal absorption and bioavailability of traditional Chinese medicines: a review of recent experimental progress and implication for quality control. J Pharm Pharmacol. 2013;65:621–33.
Ulbrich K, Reichardt N, Braune A, Kroh LW, Blaut M, Rohn S. The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavo-nifractor plautii. Food Res Int. 2015;67:349–55.
Kang MJ, Kim HG, Kim JS, Oh DG, Um YJ, Seo CS, et al. The effect of gut microbiota on drug metabolism. Expert Opin Drug Metab Toxicol. 2013;9:1295–308.
Wilson ID, Nicholson JK. Gutmicrobiomeinteractionswithdrugmetabolism, efficacy, and toxicity. Transl Res. 2017;179:204–22.
Jung IH, Lee JH, Hyun YJ, Kim DH. Metabolism of ginsenoside Rb1 by human intestinal microflora and cloning of its metabolizing beta-D-glucosidase from bifidobacterium longum H-1. Biol Pharm Bull. 2012;35:573–81.
Braune A, Engst W, Blaut M. Identification and functional expression of genes encoding flavonoid O- and C-glycosidases in intestinal bacteria. Environ Microbiol. 2016;18:2117–29.
Adamsson I, Edlund C, Nord CE. Microbial ecology and treatment of Heli-cobacter pylori infections: review. J Chemother. 2000;12:5–16.
Neuhann HF, Swai B, Carpenter CF, Shao O. Efficacy of different antibiotic regimens for eradication treatment of Helicobacter pylori infection in peptic ulcer disease in Tanzanian patients. Trop Doct. 2003;33:225–28.
Vale FF, Oleastro M. Overview of the phytomedicine approaches against Helicobacter pylori. World J Gastroenterol. 2014;20:5594–609.
Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinalhomeostasis. Cell. 2004;118:229–41.
Emal D, Rampanelli E, Stroo I, Butter LM, Teske GJ, Claessen N, et al. Depletion of gut microbiota protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2016;28:1450–61.
Ge XL, Ding C, Zhao W, Xu LZ, Tian HL, Gong JF, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J Transl Med. 2017;15:13.
Grasa L, Abecia L, Forcén R, Castro M, de Jalón JA, Latorre E, et al. Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microb Ecol. 2015;70:835–48.
Jin YX, Wu Y, Zeng ZY, Jin CY, Wu SS, Wang YY, et al. From the cover: exposure to oral antibiotics induces gut microbiota dysbiosis associated with lipid metabolism dysfunction and low-grade inflammation in mice. Toxicol Sci. 2016;154:140–52.
Burton PS, Goodwin JT, Vidmar TJ, Amore BM. Predicting drug absorption: how nature made it a difficult problem. J Pharmacol Exp Ther. 2002;303:889–95.
Pai MP, Momary KM, Rodvold KA. Antibiotic drug interactions. Med Clin North Am. 2006;90:1223–55.
Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–38.
He JX, Akao T, Tani T. Influence of co-administered antibiotics on the pharmacokinetic fate in rats of paeoniflorin and its active metabolite paeonimetabolin-I from Shaoyao-Gancao-tang. J Pharm Pharmacol. 2003;55:313–21.
Cao WL, Wang XG, Li HJ, Shi XL, Fan WC, Zhao SH, et al. Studies on metabolism of total glucosides of paeony from Paeoniae Radix Alba in rats by UPLC-Q-TOF-MS/MS. Biomed Chromatogr. 2015;29:1769–79.
Donaldson GP, Lee SM, Mazmanian SK. Gutbiogeographyofthebacterial microbiota. Nat Rev Microbiol. 2016;14:20–32.
Yang XD, Wang C, Zhou P, Yu J, Asenso J, Ma Y, et al. Absorption characteristic of paeoniflorin-6’-O-benzene sulfonate (CP-25) in in situ single-pass intestinal perfusion in rats. Xenobiotica. 2016;46:775–83.
Kim DH, Hong SW, Kim BT, Bae EA, Park HY, Han MJ. Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch Pharm Res. 2000;23:172–77.
Acknowledgements
This study was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA12050306), the Youth Innovation Promotion Association CAS and the Active Natural Product Project from CAS ZSTH-009.
Author information
Authors and Affiliations
Contributions
J.L., S.Y., and M.L. designed this study; M.L., J.Y., W.-J.H., and C.-Q.K. carried out the experiments; M.L. and W.-J.H. analyzed the data; M.L., J.L., and S.Y. prepared the manuscript; S.Y., J.L., Y.Y., D.-F.Z., Y.-F.Z., and G.-R.Z. critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liu, M., Yuan, J., Hu, Wj. et al. Pretreatment with broad-spectrum antibiotics alters the pharmacokinetics of major constituents of Shaoyao-Gancao decoction in rats after oral administration. Acta Pharmacol Sin 40, 288–296 (2019). https://doi.org/10.1038/s41401-018-0011-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0011-0
Keywords
This article is cited by
-
Gut microbiota and host cytochrome P450 characteristics in the pseudo germ-free model: co-contributors to a diverse metabolic landscape
Gut Pathogens (2023)
-
Konjac flour-mediated gut microbiota alleviates insulin resistance and improves placental angiogenesis of obese sows
AMB Express (2023)
-
Pharmacokinetics-based identification of pseudoaldosterogenic compounds originating from Glycyrrhiza uralensis roots (Gancao) after dosing LianhuaQingwen capsule
Acta Pharmacologica Sinica (2021)