Abstract
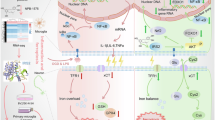
Although naloxone has been documented to exert neuroprotection in animal model of cerebral ischemia, the mechanism is not well understood. In this present study we investigated whether naloxone affected the mitochondrial apoptotic pathway in ischemic brain injury of rats. SD rats were subjected to a permanent middle cerebral artery occlusion surgery, and received naloxone (0.5, 1, 2 mg/kg, i.v.) immediately after ischemia. Neurological deficits were evaluated 24 h after ischemia using the McGraw Stroke Index, and then the rats were killed, and the brains were collected for further analyses. We show that naloxone treatment dose-dependently decreased the infarction volume and morphological injury, improved motor behavioral function, and markedly curtailed brain edema. Furthermore, naloxone administration significantly inhibited the nuclear translocation of NF-κB p65 and decreased the levels of nuclear NF-κB p65 in the ischemic penumbra. Naloxone administration also dose-dependently increased the NF-κB inhibitory protein (IκBα) levels and attenuated phosphorylated NIK and IKKα levels in the ischemic penumbra. In addition, naloxone administration dose-dependently increased Bcl-2 levels, decreased Bax levels, stabilized the mitochondrial transmembrane potential, and inhibited cytochrome c release and caspase 3 and caspase 9 activation. These results indicate that the neuroprotective effects of naloxone against ischemic brain injury involve the inhibition of NF-κB activation via the suppression of the NIK/IKKα/IκBα pathway and the obstruction of the mitochondrial apoptotic pathway in neurons.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhou N, Fu Y, Wang Y, Chen P, Meng H, Guo S, et al. p27 kip1 haplo-insufficiency improves cardiac function in early-stages of myocardial infarction by protecting myocardium and increasing angiogenesis by promoting IKK activation. Sci Rep. 2014;4:5978–88.
Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–87.
Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:331–39.
Chen CJ, Liao SL, Chen WY, Hong JS, Kuo JS. Cerebral ischemia/reperfusion injury in rat brain: effects of naloxone. Neuroreport. 2001;12:1245–49.
Wang X, Jiang CM, Wan HY, Wu JL, Quan WQ, Wu KY, et al. Neuroprotection against permanent focal cerebral ischemia by ginkgolides A and B is associated with obstruction of the mitochondrial apoptotic pathway via inhibition of c-Jun N-terminal kinase in rats. J Neurosci Res. 2014;92:232–42.
Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61:321–30.
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38:1167–86.
Gooshe M, Abdolghaffari AH, Aleyasin AR, Chabouk L, Tofigh S, Hassanzadeh GR, et al. Hypoxia/ischemia a key player in early post stroke seizures: modulation by opioidergic and nitrergic systems. Eur J Pharmacol. 2015;746:6–13.
Liao SL, Chen WY, Raung SL, Chen CJ. Neuroprotection of naloxone against ischemic injury in rats: role of mu receptor antagonism. Neurosci Lett. 2003;345:169–72.
Chen CJ, Cheng FC, Liao SL, Chen WY, Lin NN, Kuo JS. Effects of naloxone on lactate, pyruvate metabolism and antioxidant enzyme activity in rat cerebral ischemia/reperfusion. Neurosci Lett. 2000;287:113–16.
D’Ignazio L, Bandarra D, Rocha S. NF-kappaB and HIF crosstalk in immune responses. FEBS J. 2016;283:413–24.
Cildir G, Low KC, Tergaonkar V. Noncanonical NF-kappaB signaling in health and disease. Trends Mol Med. 2016;22:414–29.
Ridder DA, Schwaninger M. NF-kappaB signaling in cerebral ischemia. Neuroscience. 2009;158:995–1006.
Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–40.
Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, et al. Neuronal activation of NF-kappaB contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:30–40.
Wang X, Qin ZH, Shi H, Savitz SI, Qin AP, Jiang Y, et al. Protective effect of Ginkgolids (A+B) is associated with inhibition of NIK/IKK/IkappaB/NF-kappaB signaling pathway in a rat model of permanent focal cerebral ischemia. Brain Res. 2008;1234:8–15.
Mcgraw CP. Experimental cerebral infarctioneffects of pentobarbital in Mongolian gerbils. Arch Neurol. 1977;34:334–6.
Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, et al. Neuroprotective effect of WIN 55,212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol. 2010;645:102–7.
Liu Y, Wang D, Wang H, Qu Y, Xiao X, Zhu Y. The protective effect of HET0016 on brain edema and blood-brain barrier dysfunction after cerebral ischemia/reperfusion. Brain Res. 2014;1544:45–53.
Scholzke MN, Potrovita I, Subramaniam S, Prinz S, Schwaninger M. Glutamate activates NF-kappaB through calpain in neurons. Eur J Neurosci. 2003;18:3305–10.
Xiao S, Li D, Zhu HQ, Song MG, Pan XR, Jia PM, et al. RIG-G as a key mediator of the antiproliferative activity of interferon-related pathways through enhancing p21 and p27 proteins. Proc Natl Acad Sci USA. 2006;103:16448–53.
Hatok J, Racay P. Bcl-2 family proteins: master regulators of cell survival. Biomol Concepts. 2016;7:259–70.
Vaux DL. Apoptogenic factors released from mitochondria. Biochim Biophys Acta. 2011;1813:546–50.
Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58.
Kristiansen M, Ham J. Programmed cell death during neuronal development: the sympathetic neuron model. Cell Death Differ. 2014;21:1025–35.
Carter BZ, Mak DH, Schober WD, McQueen T, Harris D, Estrov Z, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–7.
Harari OA, Liao JK. NF-kappaB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2017;1207:32–40.
Guan T, Liu Q, Qian Y, Yang H, Kong J, Kou J, et al. Ruscogenin reduces cerebral ischemic injury via NF-kappaB-mediated inflammatory pathway in the mouse model of experimental stroke. Eur J Pharmacol. 2013;714:303–11.
Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57.
Malik S, Sharma AK, Bharti S, Nepal S, Bhatia J, Nag TC, et al. In vivo cardioprotection by pitavastatin from ischemic-reperfusion injury through suppression of IKK/NF-kappaB and upregulation of pAkt-e-NOS. J Cardiovasc Pharmacol. 2011;58:199–206.
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305.
Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59.
Razani B, Reichardt AD, Cheng G. Non-canonical NF-kappaB signaling activation and regulation: principles and perspectives. Immunol Rev. 2011;244:44–54.
Nijboer CH, Heijnen CJ, Groenendaal F, May MJ, van Bel F, Kavelaars A. Strong neuroprotection by inhibition of NF-kappaB after neonatal hypoxia- ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–37.
Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin Cell Dev Biol. 2017;72:152–62.
Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–96.
Shakeri R, Kheirollahi A, Davoodi J. Apaf-1: regulation and function in cell death. Biochimie. 2017;135:111–25.
Acknowledgements
This work was supported by the Research Program of the Committee of Science and Technology of Putuo District, Shanghai (PKW15102), National Natural Science Foundation of China Grants (81272603; 81472179), the Shanghai Shenkang Program (SHDC22014008) and the Three-year Planning for Strengthening the Construction of the Public Health System in Shanghai (2015-2017) (15GWZK0301).
Author contributions
DL and C-mJ designed the study and edited the manuscript; XW contributed to the analysis, interpretation, manuscript writing, and final approval of the manuscript; Z-jS, J-lW and W-qQ performed the research; W-dX and HC contributed new reagents, analytic tools, and manuscript writing; All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Sun, Zj., Wu, Jl. et al. Naloxone attenuates ischemic brain injury in rats through suppressing the NIK/IKKα/NF-κB and neuronal apoptotic pathways. Acta Pharmacol Sin 40, 170–179 (2019). https://doi.org/10.1038/s41401-018-0053-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0053-3
Keywords
This article is cited by
-
Chloroquine protects against mesenteric ischemia: insights into the role of nitrergic and opioidergic systems
Inflammopharmacology (2025)
-
NF-κB, A Potential Therapeutic Target in Cardiovascular Diseases
Cardiovascular Drugs and Therapy (2023)
-
Naloxone’s dose-dependent displacement of [11C]carfentanil and duration of receptor occupancy in the rat brain
Scientific Reports (2022)
-
The left–right side-specific endocrine signaling in the effects of brain lesions: questioning of the neurological dogma
Cellular and Molecular Life Sciences (2022)
-
Cardiovascular effects of antiobesity drugs: are the new medicines all the same?
International Journal of Obesity Supplements (2020)