Abstract
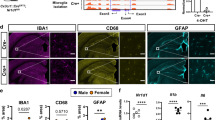
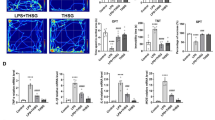
REV-ERBα, the NR1D1 (nuclear receptor subfamily 1, group D, member 1) gene product, is a dominant transcriptional silencer that represses the expression of genes involved in numerous physiological functions, including circadian rhythm, inflammation, and metabolism, and plays a crucial role in maintaining immune functions. Microglia-mediated neuroinflammation is tightly associated with various neurodegenerative diseases and psychiatric disorders. However, the role of REV-ERBα in neuroinflammation is largely unclear. In this study, we investigated whether and how pharmacological activation of REV-ERBα affected lipopolysaccharide (LPS)-induced neuroinflammation in mouse microglia in vitro and in vivo. In BV2 cells or primary mouse cultured microglia, application of REV-ERBα agonist GSK4112 or SR9011 dose-dependently suppressed LPS-induced microglial activation through the nuclear factor kappa B (NF-κB) pathway. In BV2 cells, pretreatment with GSK4112 inhibited LPS-induced phosphorylation of the inhibitor of NF-κB alpha (IκBα) kinase (IκK), thus restraining the phosphorylation and degradation of IκBα, and blocked the nuclear translocation of p65, a NF-κB subunit, thereby suppressing the expression and secretion of the proinflammatory cytokines, such as interleukin 6 (IL-6) and tumor necrosis factor α (TNFα). Moreover, REV-ERBα agonist-induced inhibition on neuroinflammation protected neurons from microglial activation-induced damage, which were also demonstrated in mice with their ventral midbrain microinjected with GSK4112, and then stimulated with LPS. Our results reveal that enhanced REV-ERBα activity suppresses microglial activation through the NF-κB pathway in the central nervous system.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34.
Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12.
Lin JR, Fang SC, Tang SS, Hu M, Long Y, Ghosh A, et al. Hippocampal CysLT1R knockdown or blockade represses LPS-induced depressive behaviors and neuroinflammatory response in mice. Acta Pharmacol Sin. 2017;38:477–87.
Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86.
Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–8.
Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–9.
Meng QJ, McMaster A, Beesley S, Lu WQ, Gibbs J, Parks D, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–35.
Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–5.
Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, et al. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–17.
Xia Q, Hu Q, Wang H, Yang H, Gao F, Ren H, et al. Induction of COX-2-PGE2 synthesis by activation of the MAPK/ERK pathway contributes to neuronal death triggered by TDP-43-depleted microglia. Cell Death Dis. 2015;6:e1702.
Guo D, Zhang S, Sun H, Xu X, Hao Z, Mu C, et al. Tyrosine hydroxylase down-regulation after loss of Abelson helper integration site 1 (AHI1) promotes depression via the circadian clock pathway in mice. J Biol Chem. 2018;293:5090–101.
Yu YX, Li YP, Gao F, Hu QS, Zhang Y, Chen D, et al. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol Sin. 2016;37:1178–89.
Fu K, Wang Y, Guo D, Wang G, Ren H. Familial Parkinson’s disease-associated L166P mutant DJ-1 is cleaved by mitochondrial serine protease Omi/HtrA2. Neurosci Bull. 2017;33:685–94.
Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–9.
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–16.
Li ST, Dai Q, Zhang SX, Liu YJ, Yu QQ, Tan F, et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-kappaB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 2018 Jan 11. https://doi.org/10.1038/aps.2017.143.
Huang Q, Wang T, Wang HY. Ginsenoside Rb2 enhances the anti-inflammatory effect of omega-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol Sin. 2017;38:192–200.
Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. 2006;281:17023–33.
Sun X, Li L, Ma HG, Sun P, Wang QL, Zhang TT, et al. Bisindolylmaleimide alkaloid BMA-155Cl induces autophagy and apoptosis in human hepatocarcinoma HepG-2 cells through the NF-kappaB p65 pathway. Acta Pharmacol Sin. 2017;38:524–38.
Ren H, Fu K, Wang D, Mu C, Wang G. Oxidized DJ-1 interacts with the mitochondrial protein BCL-XL. J Biol Chem. 2011;286:35308–17.
Zaman F, Chrysis D, Huntjens K, Chagin A, Takigawa M, Fadeel B, et al. Dexamethasone differentially regulates Bcl-2 family proteins in human proliferative chondrocytes: role of pro-apoptotic Bid. Toxicol Lett. 2014;224:196–200.
Hampton T. Circadian clock boosts stress-response genes during aging. JAMA. 2017;317:1307.
Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553:351–5.
Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–8.
Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:12662–7.
Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–7.
Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–13.
Burris TP, Busby SA, Griffin PR. Targeting orphan nuclear receptors for treatment of metabolic diseases and autoimmunity. Chem Biol. 2012;19:51–9.
Grant D, Yin L, Collins JL, Parks DJ, Orband-Miller LA, Wisely GB, et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem Biol. 2010;5:925–32.
Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, et al. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology. 2010;151:3015–25.
Morioka N, Tomori M, Zhang FF, Saeki M, Hisaoka-Nakashima K, Nakata Y. Stimulation of nuclear receptor REV-ERBs regulates tumor necrosis factor-induced expression of proinflammatory molecules in C6 astroglial cells. Biochem Biophys Res Commun. 2016;469:151–7.
Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–65.
Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci USA. 2015;112:7231–6.
Billon C, Sitaula S, Burris TP. Inhibition of RORalpha/gamma suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metabol. 2016;5:997–1005.
Stujanna EN, Murakoshi N, Tajiri K, Xu D, Kimura T, Qin R, et al. Rev-erb agonist improves adverse cardiac remodeling and survival in myocardial infarction through an anti-inflammatory mechanism. PLoS ONE. 2017;12:e0189330.
Acknowledgements
This work was supported by the National Key Scientific R&D Program of China (No. 2016YFC1306000), the National Natural Science Foundation of China (No's. 31471012, 81761148024, and 31330030), Suzhou Clinical Research Center of Neurological Disease (No. Szzx201503) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author contribution
Dong-kai Guo performed most of the experiments and analyzed the data as well as drafted and revised manuscript; Yao Zhu supplemented experiments and revised the manuscript; Hong-yang Sun performed animal experiments; Xing-yun Xu performed immunoblot assays; Shun Zhang performed immunoblot assays; Zong-bing Hao performed immunoblot assays; Guang-hui Wang revised the manuscript; Chen-chen Mu performed immunoblot analyses; Hai-gang Ren analyzed the data, drafted the manuscript, and discussed the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Guo, Dk., Zhu, Y., Sun, Hy. et al. Pharmacological activation of REV-ERBα represses LPS-induced microglial activation through the NF-κB pathway. Acta Pharmacol Sin 40, 26–34 (2019). https://doi.org/10.1038/s41401-018-0064-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0064-0
Keywords
This article is cited by
-
Unraveling the Role of METTL3 in Helicobacter pylori-induced gastritis via m6A-CXCL1/NF-κB modulation
Cell Death & Disease (2025)
-
Loss of NgBR causes neuronal damage through decreasing KAT7-mediated RFX1 acetylation and FGF1 expression
Cellular and Molecular Life Sciences (2025)
-
Chronic Exercise Protects Against Cognitive Deficits in an Alzheimer’s Disease Model by Enhancing Autophagy and Reducing Mitochondrial Abnormalities
Molecular Neurobiology (2025)
-
Circadian rhythm disruption modulates enteric neural precursor cells differentiation leading to gastrointestinal motility dysfunction via the NR1D1/NF-κB axis
Journal of Translational Medicine (2024)
-
MAVS signaling shapes microglia responses to neurotropic virus infection
Journal of Neuroinflammation (2024)