Abstract
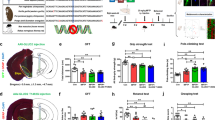
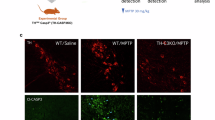
Neuroprotection targeting mitochondrial dysfunction has been proposed as an important therapeutic strategy for Parkinson’s disease. Ganoderma lucidum (GL) has emerged as a novel agent that protects neurons from oxidative stress. However, the detailed mechanisms underlying GL-induced neuroprotection have not been documented. In this study, we investigated the neuroprotective effects of GL extract (GLE) and the underlying mechanisms in the classic MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced mouse model of PD. Mice were injected with MPTP to induce parkinsonism. Then the mice were administered GLE (400 mg kg−1 d−1, ig) for 4 weeks. We observed that GLE administration significantly improved locomotor performance and increased tyrosine hydroxylase expression in the substantia nigra pars compact (SNpc) of MPTP-treated mice. In in vitro study, treatment of neuroblastoma neuro-2a cells with 1-methyl-4-phenylpyridinium (MPP+, 1 mmol/L) caused mitochondrial membrane potential collapse, radical oxygen species accumulation, and ATP depletion. Application of GLE (800 μg/mL) protected neuroblastoma neuro-2a cells against MPP+ insult. Application of GLE also improved mitochondrial movement dysfunction in cultured primary mesencephalic neurons. In addition, GLE counteracted the decline in NIX (also called BNIP3L) expression and increase in the LC3-II/LC3-I ratio evoked by MPP+. Moreover, GLE reactivated MPP+-inhibited AMPK, mTOR, and ULK1. Similarly, GLE was sufficient to counteract MPP+-induced inhibition of PINK1 and Parkin expression. GLE suppressed MPP+-induced cytochrome C release and activation of caspase-3 and caspase-9. In summary, our results provide evidence that GLE ameliorates parkinsonism pathology via regulating mitochondrial function, autophagy, and apoptosis, which may involve the activation of both the AMPK/mTOR and PINK1/Parkin signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Hirsch EC, Jenner P, Przedborski S. Pathogenesis of Parkinson’s disease. Mov Disord. 2013;28:24–30.
Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222.
Exner N, Lutz AK, Haass C, Winklhofer KF. Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences. EMBO J. 2012;31:3038–62.
Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol. 2013;106-107:17–32.
Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–7.
Zuo L, Motherwell MS. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene. 2013;532:18–23.
Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009357.
Zhang H, Duan C, Yang H. Defective autophagy in Parkinson’s disease: lessons from genetics. Mol Neurobiol. 2015;51:89–104.
Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, et al. Lysosomal impairment in Parkinson’s disease. Mov Disord. 2013;28:725–32.
Foo JN, Liany H, Bei JX, Yu XQ, Liu J, Au WL, et al. Rare lysosomal enzyme gene SMPD1 variant (p.R591C) associates with Parkinson’s disease. Neurobiol Aging. 2013;34:2890 e13–5.
Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–98.
Vilarino-Guell C, Wider C, Ross OA, Dachsel JC, Kachergus JM, Lincoln SJ, et al. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–7.
Du TT, Wang L, Duan CL, Lu LL, Zhang JL, Gao G, et al. GBA deficiency promotes SNCA/alpha-synuclein accumulation through autophagic inhibition by inactivated PPP2A. Autophagy. 2015;11:1803–20.
Tang FL, Erion JR, Tian Y, Liu W, Yin DM, Ye J, et al. VPS35 in dopamine neurons is required for endosome-to-golgi retrieval of Lamp2a, a receptor of chaperone-mediated autophagy that is critical for alpha-synuclein degradation and prevention of pathogenesis of Parkinson’s disease. J Neurosci. 2015;35:10613–28.
Salawu FK, Danburam A, Olokoba AB. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Niger J Med. 2010;19:126–31.
Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11:25–40.
Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136:374–84.
Du H, Yan SS. Mitochondrial medicine for neurodegenerative diseases. Int J Biochem Cell Biol. 2010;42:560–72.
Schapira AH, Patel S. Targeting mitochondria for neuroprotection in Parkinson disease. JAMA Neurol. 2014;71:537–8.
Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71:543–52.
Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, et al. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. J Am Med Assoc. 2015;313:584–93.
Batra P, Sharma AK, Khajuria R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): a bitter mushroom with amazing health benefits. Int J Med Mushrooms. 2013;15:127–43.
Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macrofungus. Curr Pharm Biotechnol. 2009;10:717–42.
Wachtel-Galor S, Yuen J, Buswell JA, Benzie IFF. Ganoderma lucidum (Lingzhi or Reishi): a medicinal mushroom.In:Benzie IFF, Wachtel-Galor S, editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 9.
Zhang R, Xu S, Cai Y, Zhou M, Zuo X, Chan P. Ganoderma lucidum protects dopaminergic neuron degeneration through inhibition of microglial activation. Evid Based Complement Altern Med. 2011;2011:156810.
Bhardwaj N, Katyal P, Sharma AK. Suppression of inflammatory and allergic responses by pharmacologically potent fungus Ganoderma lucidum. Recent Pat Inflamm Allergy Drug Discov. 2014;8:104–17.
Ma HT, Hsieh JF, Chen ST. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry. 2015;114:109–13.
Chen LW, Wang YQ, Wei LC, Shi M, Chan YS. Chinese herbs and herbal extracts for neuroprotection of dopaminergic neurons and potential therapeutic treatment of Parkinson’s disease. CNS Neurol Disord Drug Targets. 2007;6:273–81.
Lai CS, Yu MS, Yuen WH, So KF, Zee SY, Chang RC. Antagonizing beta-amyloid peptide neurotoxicity of the anti-aging fungus Ganoderma lucidum. Brain Res. 2008;1190:215–24.
Shen B, Truong J, Helliwell R, Govindaraghavan S, Sucher NJ. An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Complement Altern Med. 2013;13:373.
Zhao HB, Lin SQ, Liu JH, Lin ZB. Polysaccharide extract isolated from ganoderma lucidum protects rat cerebral cortical neurons from hypoxia/reoxygenation injury. J Pharmacol Sci. 2004;95:294–8.
Zhou Y, Qu ZQ, Zeng YS, Lin YK, Li Y, Chung P, et al. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp Toxicol Pathol. 2012;64:673–80.
Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, et al. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol. 2010;131:154–64.
Guo SS, Cui XL, Rausch WD. Ganoderma lucidum polysaccharides protect against MPP(+) and rotenone-induced apoptosis in primary dopaminergic cell cultures through inhibiting oxidative stress. Am J Neurodegener Dis. 2016;5:131–44.
Gokce EC, Kahveci R, Atanur OM, Gurer B, Aksoy N, Gokce A, et al. Neuroprotective effects of Ganoderma lucidum polysaccharides against traumatic spinal cord injury in rats. Injury. 2015;46:2146–55.
Zhang W, Zhang Q, Deng W, Li Y, Xing G, Shi X, et al. Neuroprotective effect of pretreatment with ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus. Neural Regen Res. 2014;9:1446–52.
Ding H, Zhou M, Zhang RP, Xu SL. Ganoderma lucidum extract protects dopaminergic neurons through inhibiting the production of inflammatory mediators by activated microglia. Sheng Li Xue Bao. 2010;62:547–54.
Arduino DM, Esteves AR, Cardoso SM. Mitochondria drive autophagy pathology via microtubule disassembly: a new hypothesis for Parkinson disease. Autophagy. 2013;9:112–4.
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49.
Perier C, Bove J, Vila M. Mitochondria and programmed cell death in Parkinson’s disease: apoptosis and beyond. Antioxid Redox Signal. 2012;16:883–95.
Millecamps S, Julien J-P. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–76.
Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125:2095–104.
Sheng ZH. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol. 2014;204:1087–98.
Kim-Han JS, Antenor-Dorsey JA, O’Malley KL. The parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–21.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23.
Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–7.
Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11.
Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52:381–400.
Kazlauskaite A, Muqit MM. PINK1 and Parkin—mitochondrial interplay between phosphorylation and ubiquitylation in Parkinson’s disease. FEBS J. 2015;282:215–23.
Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci. 2014;37:315–24.
Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid Redox Signal. 2011;14:1929–38.
Barodia SK, Creed RB, Goldberg MS. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res Bull. 2016;133:51–9.
Boh B, Berovic M, Zhang J, Zhibin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol Annu Rev. 2007;13:265–301.
Sliva D. Cellular and physiological effects of Ganoderma lucidum (Reishi). Mini Rev Med Chem. 2004;4:873–9.
Boh B. Ganoderma lucidum: a potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Pat Anticancer Drug Discov. 2013;8:255–87.
Ferreira IC, Heleno SA, Reis FS, Stojkovic D, Queiroz MJ, Vasconcelos MH, et al. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry. 2015;114:38–55.
Xu Z, Chen X, Zhong Z, Chen L, Wang Y. Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med. 2011;39:15–27.
Li X, Gong H, Yang S, Yang L, Fan Y, Zhou Y. Pectic bee pollen polysaccharide from Rosa rugosa alleviates diet-induced hepatic steatosis and insulin resistance via induction of AMPK/mTOR-mediated autophagy. Molecules. 2017;22:E699.
Liao LZ, Chen YL, Lu LH, Zhao YH, Guo HL, Wu WK. Polysaccharide from Fuzi likely protects against starvation-induced cytotoxicity in H9c2 cells by increasing autophagy through activation of the AMPK/mTOR pathway. Am J Chin Med. 2013;41:353–67.
Lu L, Huang YF, Chen DX, Wang M, Zou YC, Wan H, et al. Astragalus polysaccharides decrease muscle wasting through Akt/mTOR, ubiquitin proteasome and autophagy signalling in 5/6 nephrectomised rats. J Ethnopharmacol. 2016;186:125–35.
Acknowledgements
This study was supported by grants from the China Postdoctoral Science Foundation (2015M581133) and Beijing Postdoctoral Research Foundation (2015ZZ-63); from Beijing Municipal Administration of Hospitals’ Mission Plan, Code: SML20150803 and Beijing Municipal Science & Technology Commission No. Z171100000117013 to Dr. Piu Chan; and from The National Key R&D Program of China (2016YFC1306000) to Dr. Chao-dong Wang.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ren, Zl., Wang, Cd., Wang, T. et al. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol Sin 40, 441–450 (2019). https://doi.org/10.1038/s41401-018-0077-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0077-8
keywords
This article is cited by
-
Mitochondrial dynamics reveal potential to facilitate axonal regeneration after spinal cord injury
Journal of Translational Medicine (2025)
-
GSK-126 Attenuates Cell Apoptosis in Ischemic Brain Injury by Modulating the EZH2-H3K27me3-Bcl2l1 Axis
Molecular Neurobiology (2024)
-
Neuroprotective effects of morroniside from Cornus officinalis sieb. Et zucc against Parkinson’s disease via inhibiting oxidative stress and ferroptosis
BMC Complementary Medicine and Therapies (2023)
-
Linking Heat Shock Protein 70 and Parkin in Parkinson’s Disease
Molecular Neurobiology (2023)
-
First report of chemical composition and cytotoxicity evaluation of Foraminispora rugosa basidiomata from Brazil
Botanical Studies (2022)