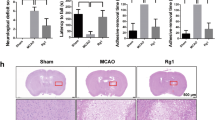
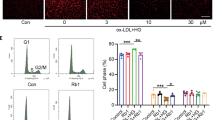
Abstract
Ginsenoside Rg1 (Rg1), a saponin extracted from Panax ginseng, has been well documented to be effective against ischemic/reperfusion (I/R) neuronal injury. However, the underlying mechanisms remain obscure. In the present study, we investigated the roles of Nrf2 and miR-144 in the protective effects of Rg1 against I/R-induced neuronal injury. In OGD/R-treated PC12 cells, Rg1 (0.01–1 μmol/L) dose-dependently attenuated the cell injury accompanied by prolonging nuclear accumulation of Nrf2, enhancing the transcriptional activity of Nrf2, as well as promoting the expression of ARE-target genes. The activation of the Nrf2/ARE pathway by Rg1 was independent of disassociation with Keap1, but resulted from post-translational regulations. Knockdown of Nrf2 abolished all the protective changes of Rg1 in OGD/R-treated PC12 cells. Furthermore, Rg1 treatment significantly decreased the expression of miR-144, which downregulated Nrf2 production by targeting its 3’-untranlated region after OGD/R. Knockdown of Nrf2 had no effect on the expression of miR-144, suggesting that miR-144 was an upstream regulator of Nrf2. We revealed that there was a direct binding between Nrf2 and miR-144 in PC12 cells. Application of anti-miR-144 occluded the activation of the Nrf2/ARE pathway by Rg1 in OGD/R-treated PC12 cells. In tMCAO rats, administration of Rg1 (20 mg/kg) significantly alleviated ischemic injury, and activated Nrf2/ARE pathway. The protective effects of Rg1 were abolished by injecting of AAV-HIF-miR-144-shRNA into the predicted ischemic penumbra. In conclusion, our results demonstrate that Rg1 alleviates oxidative stress after I/R through inhibiting miR-144 activity and subsequently promoting the Nrf2/ARE pathway at the post-translational level.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
29 April 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41401-024-01272-1
References
Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–72.
Shin EJ, Shin SW, Nguyen TT, Park DH, Wie MB, Jang CG, et al. Ginsenoside Re rescues methamphetamine-induced oxidative damage, mitochondrial dysfunction, microglial activation, and dopaminergic degeneration by inhibiting the protein kinase Cdelta gene. Mol Neurobiol. 2014;49:1400–21.
Wang J, Li D, Hou J, Lei H. Protective effects of geniposide and ginsenoside Rg1 combination treatment on rats following cerebral ischemia are mediated via microglial microRNA1555p inhibition. Mol Med Rep. 2018;17:3186–93.
Li Y, Suo L, Liu Y, Li H, Xue W. Protective effects of ginsenoside Rg1 against oxygen-glucose-deprivation-induced apoptosis in neural stem cells. J Neurol Sci. 2017;373:107–12.
Zhou Y, Li HQ, Lu L, Fu DL, Liu AJ, Li JH, et al. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine. 2014;21:998–1003.
Xie CL, Wang WW, Xue XD, Zhang SF, Gan J, Liu ZG. A systematic review and meta-analysis of Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep. 2015;5:7790.
Lin M, Sun W, Gong W, Ding Y, Zhuang Y, Hou Q. Ginsenoside Rg1 protects against transient focal cerebral ischemic injury and suppresses its systemic metabolic changes in cerabral injury rats. Acta Pharm Sin B. 2015;5:277–84.
Shimizu Y, Nicholson CK, Lambert JP, Barr LA, Kuek N, Herszenhaut D, et al. Sodium sulfide attenuates ischemic-induced heart failure by enhancing proteasomal function in an Nrf2-dependent manner. Circ Heart Fail. 2016;9:e002368.
Wakayama K, Shimamura M, Suzuki JI, Watanabe R, Koriyama H, Akazawa H, et al. Angiotensin II peptide vaccine protects ischemic brain through reducing oxidative stress. Stroke. 2017;48:1362–8.
Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, et al. An antagomir to MicroRNA-106b-5p ameliorates cerebral ischemia and reperfusion Injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54:2901–21.
Li P, Stetler RA, Leak RK, Shi Y, Li Y, Yu W, et al. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology. 2017;134:208–17.
Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51:967–77.
Radermacher KA, Wingler K, Langhauser F, Altenhofer S, Kleikers P, Hermans JJ, et al. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid Redox Signal. 2013;18:1418–27.
Zheng Y, Wang Y, Zhang X, Tan Y, Peng S, Chen L, et al. C19, a C-terminal peptide of CKLF1, decreases inflammation and proliferation of dermal capillaries in psoriasis. Sci Rep. 2017;7:13890.
Carbone F, Teixeira PC, Braunersreuther V, Mach F, Vuilleumier N, Montecucco F. Pathophysiology and treatments of oxidative injury in Ischemic stroke: focus on the phagocytic NADPH oxidase 2. Antioxid Redox Signal. 2015;23:460–89.
Takayasu Y, Nakaki J, Kawasaki T, Koda K, Ago Y, Baba A, et al. Edaravone, a radical scavenger, inhibits mitochondrial permeability transition pore in rat brain. J Pharmacol Sci. 2007;103:434–7.
Tang M, Feng W, Zhang Y, Zhong J, Zhang J. Salvianolic acid B improves motor function after cerebral ischemia in rats. Behav Pharmacol. 2006;17:493–8.
Wu G, Zhu L, Yuan X, Chen H, Xiong R, Zhang S, et al. Britanin ameliorates cerebral ischemia-reperfusion injury by inducing the Nrf2 protective pathway. Antioxid Redox Signal. 2017;27:754–68.
Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G, et al. Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm Res. 2018;67:57–65.
Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, et al. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2018;28:141–63.
Tanaka N, Ikeda Y, Ohta Y, Deguchi K, Tian F, Shang J, et al. Expression of Keap1-Nrf2 system and antioxidative proteins in mouse brain after transient middle cerebral artery occlusion. Brain Res. 2011;1370:246–53.
Fujita K, Maeda D, Xiao Q, Srinivasula SM. Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc Natl Acad Sci U S A. 2011;108:1427–32.
Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–35.
Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, et al. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–22.
Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C. NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306.
Ding Y, Chen M, Wang M, Li Y, Wen A. Posttreatment with 11-keto-beta-boswellic acid ameliorates cerebral ischemia-reperfusion injury: Nrf2/HO-1 pathway as a potential mechanism. Mol Neurobiol. 2015;52:1430–9.
Guo Y, Yu S, Zhang C, Kong AN. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic Biol Med. 2015;88:337–49.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31.
Yu M, Liu Y, Zhang B, Shi Y, Cui L, Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovasc Pathol. 2015;24:375–81.
Liu L, Sun T, Liu Z, Chen X, Zhao L, Qu G, et al. Traumatic brain injury dysregulates microRNAs to modulate cell signaling in rat hippocampus. PLoS One. 2014;9:e103948.
Ji Q, Gao J, Zheng Y, Liu X, Zhou Q, Shi C, et al. Inhibition of microRNA-153 protects neurons against ischemia/reperfusion injury in an oxygen-glucose deprivation and reoxygenation cellular model by regulating Nrf2/HO-1 signaling. J Biochem Mol Toxicol 2017; 31. https://doi.org/10.1002/jbt.21905.
Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423.
Zhou C, Zhao L, Zheng J, Wang K, Deng H, Liu P, et al. MicroRNA-144 modulates oxidative stress tolerance in SH-SY5Y cells by regulating nuclear factor erythroid 2-related factor 2-glutathione axis. Neurosci Lett. 2017;655:21–7.
Chu SF, Zhang Z, Zhang W, Zhang MJ, Gao Y, Han N, et al. Upregulating the expression of survivin-HBXIP complex contributes to the protective role of IMM-H004 in transient global cerebral ischemia/reperfusion. Mol Neurobiol. 2017;54:524–40.
Gao Y, Chu S, Shao Q, Zhang M, Xia C, Wang Y, et al. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radic Res. 2017;51:1–13.
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke. 1998;29:1037–46.
Ashwal S, Tone B, Tian HR, Cole DJ, Liwnicz BH, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion in the rat pup. Pediatr Res. 1999;46:390–400.
Niu F, Song XY, Hu JF, Zuo W, Kong LL, Wang XF, et al. IMM-H004, a new coumarin derivative, improved focal cerebral ischemia via blood-brain barrier protection in rats. J Stroke Cerebrovasc Dis. 2017;26:2065–73.
Kong LL, Wang ZY, Han N, Zhuang XM, Wang ZZ, Li H, et al. Neutralization of chemokine-like factor 1, a novel C-C chemokine, protects against focal cerebral ischemia by inhibiting neutrophil infiltration via MAPK pathways in rats. J Neuroinflammation. 2014;11:112.
Zhang XS, Ha S, Wang XL, Shi YL, Duan SS, Li ZA. Tanshinone IIA protects dopaminergic neurons against 6-hydroxydopamine-induced neurotoxicity through miR-153/NF-E2-related factor 2/antioxidant response element signaling pathway. Neuroscience. 2015;303:489–502.
Sangokoya C, Telen MJ, Chi JT. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116:4338–48.
Heng Y, Zhang QS, Mu Z, Hu JF, Yuan YH, Chen NH. Ginsenoside Rg1 attenuates motor impairment and neuroinflammation in the MPTP-probenecid-induced parkinsonism mouse model by targeting alpha-synuclein abnormalities in the substantia nigra. Toxicol Lett. 2016;243:7–21.
Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F, Chen LM. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26:56–62.
Yu HT, Zhen J, Pang B, Gu JN, Wu SS. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ Sci B. 2015;16:344–54.
Li J, Cai D, Yao X, Zhang Y, Chen L, Jing P, et al. Protective effect of ginsenoside Rg1 on hematopoietic stem/progenitor cells through attenuating oxidative stress and the Wnt/beta-catenin signaling pathway in a mouse model of d-galactose-induced aging. Int J Mol Sci. 2016;17:pii:E849.
Li JP, Gao Y, Chu SF, Zhang Z, Xia CY, Mou Z, et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCI4-induced hepatic fibrosis. Acta Pharmacol Sin. 2014;35:1031–44.
Zhang R, Xu M, Wang Y, Xie F, Zhang G, Qin X. Nrf2-a promising therapeutic target for defensing against oxidative stress in stroke. Mol Neurobiol. 2017;54:6006–17.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81603315, 81730096, 81603316, 81503275, 81730093, 81873026, U1402221), the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-004), the State Key Laboratory Fund Open Project (GTZK201610), the China Postdoctoral Science Foundation (2013M540066), the PUMC Graduate Education and Teaching Reform Project (10023201600801), the Beijing Key Laboratory of New Drug Mechanisms and Pharmacological Evaluation Study (BZ0150), the opening Program of Shanxi Key Laboratory of Chinese Medicine Encephalopathy (CME-OP-2017001), and the Hunan Chinese Herbal Medicine and standardization functions Engineering Research Center (BG201701).
Author information
Authors and Affiliations
Contributions
N.-h.C. designed research; S.-f.C. and Z.Z. performed the cell experiments and Nrf2-related determination; Xin Zhou, Chen Chen, and Dan-dan Liu prepared the animal models; W.-B.H. guided the animal experiments; P.L., Z.-z.W. and Q.-d.A. determined ROS and 8-OHDG in brain slide; S.-f.C., H.-f.G., H.-S.S. and Z.-P.F. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chu, Sf., Zhang, Z., Zhou, X. et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol Sin 40, 13–25 (2019). https://doi.org/10.1038/s41401-018-0154-z
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0154-z
Keywords
This article is cited by
-
Chronic lipopolysaccharide exposure promotes cognitive impairments by activating TRPC6-AIM2 inflammasome signaling and the regulation of ginsenoside Rg1 in Trpc6−/− mice
Behavioral and Brain Functions (2025)
-
Electro-Acupuncture Therapy Alleviates Post-Stroke Insomnia by Regulating Sirt1 and the Nrf2-ARE Pathway
NeuroMolecular Medicine (2025)
-
Complementary therapies for stroke towards neurorecovery
Discover Medicine (2025)
-
Efficacy of ginsenoside Rg1 on rodent models of depression: A systematic review and meta-analysis
Psychopharmacology (2025)
-
Ginsenoside Rg1 mitigates myocardial ischemia/reperfusion injury by inhibiting NLRP3-mediated pyroptosis
In Vitro Cellular & Developmental Biology - Animal (2025)