Abstract
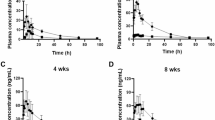
ShenMai, an intravenous injection prepared from steamed Panax ginseng roots (Hongshen) and Ophiopogon japonicus roots (Maidong), is used as an add-on therapy for coronary artery disease and cancer; saponins are its bioactive constituents. Since many saponins inhibit human organic anion-transporting polypeptides (OATP)1B, this investigation determined the inhibition potencies of circulating ShenMai saponins on the transporters and the joint potential of these compounds for ShenMai-drug interaction. Circulating saponins and their pharmacokinetics were characterized in rats receiving a 30-min infusion of ShenMai at 10 mL/kg. Inhibition of human OATP1B1/1B3 and rat Oatp1b2 by the individual saponins was investigated in vitro; the compounds’ joint inhibition was also assessed in vitro and the data was processed using the Chou–Talalay method. Plasma protein binding was assessed by equilibrium dialysis. Altogether, 49 saponins in ShenMai were characterized and graded into: 10–100 μmol/day (compound doses from ShenMai; 7 compounds), 1–10 μmol/day (17 compounds), and <1 μmol/day (25 compounds, including Maidong ophiopogonins). After dosing, circulating saponins were protopanaxadiol-type ginsenosides Rb1, Rb2, Rc, Rd, Ra1, Rg3, Ra2, and Ra3, protopanaxatriol-type ginsenosides Rg1, Re, Rg2, and Rf, and ginsenoside Ro. The protopanaxadiol-type ginsenosides exhibited maximum plasma concentrations of 2.1–46.6 μmol/L, plasma unbound fractions of 0.4–1.0% and terminal half-lives of 15.6–28.5 h (ginsenoside Rg3, 1.9 h), while the other ginsenosides exhibited 0.1–7.7 μmol/L, 20.8–99.2%, and 0.2–0.5 h, respectively. The protopanaxadiol-type ginsenosides, ginsenosides without any sugar attachment at C-20 (except ginsenoside Rf), and ginsenoside Ro inhibited OATP1B3 more potently (IC50, 0.2–3.5 µmol/L) than the other ginsenosides (≥22.6 µmol/L). Inhibition of OATP1B1 by ginsenosides was less potent than OATP1B3 inhibition. Ginsenosides Rb1, Rb2, Rc, Rd, Ro, Ra1, Re, and Rg2 likely contribute the major part of OATP1B3-mediated ShenMai-drug interaction potential, in an additive and time-related manner.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zhang M-M, Liu X-M, Li J, He L, Tripathy D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst Rev. 2007;18:CD004921.
Lam W, Bussom S, Guan F-L, Jiang Z-L, Zhang W, Gullen EA, Liu SH, Cheng YC. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2:45ra59.
Li X-L, Zhang J, Huang J, Ma A-Q, Yang J-F, Li W-M, et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of Qili Qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065–72.
Hao P-P, Jiang F, Chen Y-G, Yang J-M, Zhang K, Zhang M-X, et al. Traditional Chinese medication for cardiovascular disease. Nat Rev Cardiol. 2015;12:115–22.
Chinese Society of Critical Care Medicine. Chinese guidelines for management of severe sepsis and septic shock 2014. Chin Crit Care Med. 2015;27:401–26.
Expert Committee on Clinical Application of Glycyrrhizin Preparation in the Treatment of Liver Diseases. Expert consensus on clinical application of glycyrrhizin preparation in the treatment of liver diseases. J Clin Hepatol. 2016;32:844–52.
Song Y-L, Yao C, Shang H-C, Yao X-Q, Bai C-X. Late-breaking abstract: intravenous infusion of Chinese medicine XueBiJing significantly improved clinical outcome in severe pneumonia patients in a multiple center randomized controlled clinical trials. Eur Respir J. 2016;48:OA3323.
Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–8.
Hermann R, Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012;78:1458–77.
Stieger B, Mahdi ZM, Jager W. Intestinal and hepatocellular transporters: therapeutic effects and drug interactions of herbal supplements. Annu Rev Pharmacol Toxicol. 2017;57:399–416.
Grimstein M, Huang S-M. A regulatory science viewpoint on botanical-drug interactions. J Food Drug Anal. 2018. 10.016/j.jfda.2018.01.013.
Brantley SJ, Argikar AA, Lin YS, Nagar S, Paine MF. Herb-drug interactions: challenges and opportunities for improved predictions. Drug Metab Dispos. 2014;42:301–17.
Gufford BT, Chen G, Vergara AG, Lazarus P, Oberlies NH, Paine MF. Milk Thistle constituents inhibit raloxifene intestinal glucuronidation: a potential clinically relevant natural product-drug interaction. Drug Metab Dispos. 2015;43:353–9.
Jiang R-R, Dong J-J, Li X-X, Du F-F, Jia W-W, Xu F, et al. Molecular mechanisms governing different pharmacokinetics of ginsenosides and potential for ginsenoside-perpetrated herb-drug interactions on OATP1B3. Br J Pharmacol. 2015;172:1059–73.
Wu X, Ma J, Ye Y, Lin G. Transporter modulation by Chinese herbal medicines and its mediated pharmacokinetic herb-drug interactions. J Chromatogr B. 2016;1026:236–53.
Li Y, Revalde J, Paxton JW. The effects of dietary and herbal phytochemicals on drug transporters. Adv Drug Deliv Rev. 2017;116:45–62.
Dong J-J, Olaleye OE, Jiang R-R, Li J, Lu C, Du F-F, et al. Glycyrrhizin has a high propensity to be a victim of herb-drug interactions on hepatic OATP1B1/1B3. Br J Pharmacol. 2018;175:3486–503.
Xian S-X, Yang Z-Q, Lee J, Jiang Z-P, Ye X-H, Luo L-Y, et al. A randomized, double-blind, multicenter, placebo-controlled clinical study on the efficacy and safety of Shenmai injection in patients with chronic heart failure. J Ethnopharmacol. 2016;186:136–42.
Zhang W-L, Yan T-H, Liu B, Wang J, Zhao N-P, Shu L-X, et al. Systematic reviews and meta-analysis of ShenMai injection with chemotherapy on treatment of patients with non-small cell lung cancer. Pract Pharm Clin Remed. 2011;14:95–101.
Fan X-H, Wang Y, Cheng Y-Y. LC/MS fingerprinting of Shenmai injection: a novel approach to quality control of herbal medicines. J Pharm Biomed Anal. 2006;40:591–7.
Karmazyn M, Moey M, Gan XT. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs. 2011;71:1989–2008.
Moey M, Gan XT, Huang CX, Rajapurohitam V, Martínez-Abundis E, Lui EMK, et al. Ginseng reverse established cardiomyocytes hypertrophy and postmyocardial infarction-induced hypertrophy and heart failure. Circ Heart Fail. 2012;5:504–14.
Chen M-H, Chen X-J, Wang M, Lin L-G, Wang Y-T. Ophiopogon japonicus—a phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol. 2016;181:193–213.
Zhang A-J, Wang C-Y, Liu Q, Meng Q, Peng J-Y, Sun H-J, et al. Involvement of organic anion-transporting polypeptides in the hepatic uptake of dioscin in rats and humans. Drug Metab Dispos. 2013;41:994–1003.
Sheng J-J, Tian X-T, Xu G-L, Wu Z-T, Chen C, Wang L, et al. The hepatobiliary disposition of timosaponin b2 is highly dependent on influx/efflux transporters but not metabolism. Drug Metab Dispos. 2015;43:63–72.
Hu Z-Y, Yang J-L, Cheng C, Huang Y-H, Du F-F, Wang F-Q, et al. Combinatorial metabolism notably affects human systemic exposure to ginsenosides from orally administered extract of Panax notoginseng roots (Sanqi). Drug Metab Dispos. 2013;41:1457–69.
McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–6.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–9.
Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–61.
Guo B, Li C, Wang G-J, Chen L-S. Rapid and direct measurement of free concentrations of highly protein-bound fluoxetine and its metabolite norfluoxetine in plasma. Rapid Commun Mass Spectrom. 2006;20:39–47.
Posada MM, Bacon JA, Schneck KB, Tirona RG, Kim RB, Higgins JW, et al. Prediction of renal transporter mediated drug-drug interactions for pemetrexed using physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2015;43:325–34.
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81.
Chou TC. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70:440–6.
Xie T, Liang Y, Hao H-P, Xie JAL, Gong P, Dai C, et al. Rapid identification of ophiopogonins and ophiopogonones in Ophiopogon japonicus extract with a practical technique of mass defect filtering based on high resolution mass spectrometry. J Chromatogr A. 2012;1227:234–44.
Yang W-Z, Ye M, Qiao X, Liu C-F, Miao W-J, Bo T, et al. A strategy for efficient discovery of new natural compounds by integrating orthogonal column chromatography and liquid chromatography/mass spectrometry analysis: Its application in Panax ginseng, Panax quinquefolium and Panax notoginseng to characterize 437 potential new ginsenosides. Anal Chim Acta. 2012;739:56–66.
Shin BK, Kwon SW, Park JH. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–98.
Li F, Cheng T-F, Dong X, Li P, Yang H. Global analysis of chemical constituents in Shengmai injection using high performance liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal. 2016;117:61–72.
European Medicines Agency. Guideline on bioanalytical method validation. London, UK: European Medicines Agency. www.ema.europa.eu/docs/en_GB/ document_library/Scientific_guideline/2011/08/ WC500109686.pdf; 2012.
Food and Drug Administration Center for Drug Evaluation and Research. In vitro metabolism- and transporter-mediated drug-drug interaction studies guidance for industry (Draft Guidance). Silver Spring, MD: U.S. Food and Drug Administration. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM581965.pdf; 2017.
Amundsen R, Christensen H, Zabihyan B, Asberg A. Cyclosporine A, but not tacrolimus, shows relevant inhibition of organic anion-transporting protein 1B1-mediated transport of atorvastatin. Drug Metab Dispos. 2010;38:1499–504.
Gertz M, Cartwright CM, Hobbs MJ, Kenworthy KE, Rowland M, Houston JB, et al. Cyclosporine inhibition of hepatic and intestinal CYP3A4, uptake and efflux transporters: application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm Res. 2013;30:761–80.
Izumi S, Nozaki Y, Maeda K, Komori T, Takenaka O, Kusuhara H, et al. Investigation of the impact of substrate selection on in vitro organic anion transporting polypeptide 1B1 inhibition profiles for the prediction of drug-drug interactions. Drug Metab Dispos. 2015;43:235–47.
Shitara Y, Sugiyama Y. Preincubation-dependent and long-lasting inhibition of organic anion transporting polypeptide (OATP) and its impact on drug-drug interactions. Pharmacol Ther. 2017;177:67–80.
Yang W-Z, Qiao X, Li K, Fan J-R, Bo T, Guo D-A, et al. Identification and differentiation of Panax ginseng, Panax quinquefolium and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm Sin B. 2016;6:568–75.
Liu H-F, Yang J-L, Du F-F, Gao X-M, Ma X-T, Huang Y-H, et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–8.
Noé J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35:1308–14.
Izumi S, Nozaki Y, Komori T, Maeda K, Takenaka O, Kusano K, et al. Substrate-dependent inhibition of organic anion transporting polypeptide 1B1: comparative analysis with prototypical probe substrates estradiol-17β-glucuronide, estrone-3-sulfate, and sulfobromophthalein. Drug Metab Dispos. 2013;41:1859–66.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (Grants 81403176 and 81673582); by the National Science and Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (Grants 2017ZX09301012-006 and 2009ZX09304-002); and by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant XDA12050306). Olajide E. Olaleye is a recipient of CAS-TWAS President’s PhD Fellowship.
Author contributions
Participated in research design: CL, J-lY, and OEO. Conducted experiments: OEO, J-lY, WN, F-fD, F-qW, FX, SP, and J-lL. Performed data analysis: CL, J-lY, and OEO. Wrote or contributed to the writing of the manuscript: CL, OEO, and J-lY.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jun-lan Lu was a visiting student from Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Olaleye, O.E., Niu, W., Du, Ff. et al. Multiple circulating saponins from intravenous ShenMai inhibit OATP1Bs in vitro: potential joint precipitants of drug interactions. Acta Pharmacol Sin 40, 833–849 (2019). https://doi.org/10.1038/s41401-018-0173-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-018-0173-9
Keywords
This article is cited by
-
Ginsenoside Rg1 in neurological diseases: From bench to bedside
Acta Pharmacologica Sinica (2023)
-
Multi-compound and drug-combination pharmacokinetic research on Chinese herbal medicines
Acta Pharmacologica Sinica (2022)
-
Pharmacokinetics-based identification of pseudoaldosterogenic compounds originating from Glycyrrhiza uralensis roots (Gancao) after dosing LianhuaQingwen capsule
Acta Pharmacologica Sinica (2021)
-
Virtual screening and network pharmacology-based synergistic mechanism identification of multiple components contained in Guanxin V against coronary artery disease
BMC Complementary Medicine and Therapies (2020)
-
Multiple circulating alkaloids and saponins from intravenous Kang-Ai injection inhibit human cytochrome P450 and UDP-glucuronosyltransferase isozymes: potential drug–drug interactions
Chinese Medicine (2020)