Abstract
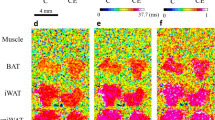
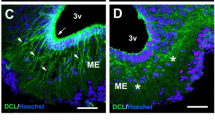
Promoting white adipose tissue (WAT) browning and enhancing brown adipose tissue (BAT) activity are attractive therapeutic strategies for obesity and its metabolic complications. Targeting sympathetic innervation in WAT and BAT represents a promising therapeutic concept. However, there are few reports on extracellular microenvironment remodeling, especially changes in nerve terminal connections. Identifying the key molecules mediating the neuro-adipose synaptic junctions is a key point. In this study, we used bioinformatics methods to identify the differentially expressed predicted secreted genes (DEPSGs) during WAT browning and BAT activation. These DEPSGs largely reflect changes of cytokines, extracellular matrix remodeling, vascularization, and adipocyte-neuronal cross-talk. We then performed functional enrichment and cellular distribution specificity analyses. The upregulated and downregulated DEPDGs during WAT browning displayed a distinctive biological pattern and cellular distribution. We listed a cluster of adipocyte-enriched DEPSGs, which might participate in the cross-talk between mature adipocytes and other cells; then identified a synaptogenic adhesion molecule, Clstn3, as the top gene expressed enriched in both mature white and brown adipocytes. Using Q-PCR and immunohistochemistry, we found significantly increased Clstn3 expression level during WAT browning and BAT activation in mice subjected to cold exposure (4 °C). We further demonstrated that treatment with isoproterenol significantly increased Clstn3 and UCP1 expression in differentiated white and beige adipocytes in vitro. In conclusion, our study demonstrates that the secretion pattern was somewhat different between WAT browning and BAT activation. We reveal that Clstn3 may be a key gene mediating the neuro-adipose junction formation or remodeling in WAT browning and BAT activation process.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Unamuno X, Gomez-Ambrosi J. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. 2018;48(9):e12997.
Expert panel on the identification, evaluation, and treatment of overweight in adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr. 1998;68:899–917.
Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–6.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63.
Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805.
Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol. 2005;25:8311–22.
Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–88.
Pope BD, Warren CR, Parker KK, Cowan CA. Microenvironmental control of adipocyte fate and function. Trends Cell Biol. 2016;26:745–55.
Jiang H, Ding X, Cao Y, Wang H, Zeng W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 2017;26:686–92.e3.
Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–29.
Muzik O, Mangner TJ, Leonard WR, Kumar A, Granneman JG. Sympathetic innervation of cold-activated brown and white fat in lean young adults. J Nucl Med. 2017;58:799–806.
Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163:84–94.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–6.
Kall L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007;35:W429–32.
Viklund H, Bernsel A, Skwark M, Elofsson A. SPOCTOPUS: a combined predictor of signal peptides and membrane protein topology. Bioinformatics. 2008;24:2928–9.
Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975;67:835–51.
Walter P, Ibrahimi I, Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981;91:545–50.
Um JW, Pramanik G, Ko JS, Song MY, Lee D, Kim H, et al. Calsyntenins function as synaptogenic adhesion molecules in concert with neurexins. Cell Rep. 2014;6:1096–109.
Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, et al. The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron. 2013;80:113–28.
Lu Z, Wang Y, Chen F, Tong H, Reddy MV, Luo L, et al. Calsyntenin-3 molecular architecture and interaction with neurexin 1alpha. J Biol Chem. 2014;289:34530–42.
Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110.
Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–67.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41:D991–5.
Burgess DJ. Gene expression: spatial characterization of proteomes. Nat Rev Genet. 2015;16:129.
Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Protein Sci. 2018;27:233–44.
The Gene Ontology Consortium. The gene ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322–6.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9.
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34.
Bai Z, Chai XR, Yoon MJ, Kim HJ, Lo KA, Zhang ZC, et al. Dynamic transcriptome changes during adipose tissue energy expenditure reveal critical roles for long noncoding RNA regulators. PLoS Biol. 2017;15:e2002176.
Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–34.
Chu DT, Malinowska E, Gawronska-Kozak B, Kozak LP. Expression of adipocyte biomarkers in a primary cell culture models reflects preweaning adipobiology. J Biol Chem. 2014;289:18478–88.
Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–31.
Mowers J, Uhm M, Reilly SM, Simon J, Leto D, Chiang SH, et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKε and TBK1. eLife. 2013;2:e01119.
Missler M, Sudhof TC, Biederer T. Synaptic cell adhesion. Cold Spring Harb Perspect Biol. 2012;4:a005694.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81471085, No. 81670778, and No. 81603476) and the Innovation Fund for PhD Students from Shanghai Jiao Tong University School of Medicine (BXJ201841).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, Sq., Niu, Q., Ju, Lp. et al. Predicted secreted protein analysis reveals synaptogenic function of Clstn3 during WAT browning and BAT activation in mice. Acta Pharmacol Sin 40, 999–1009 (2019). https://doi.org/10.1038/s41401-019-0211-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0211-2
Keywords
This article is cited by
-
Perinatal Lead Exposure Alters Calsyntenin-2 and Calsyntenin-3 Expression in the Hippocampus and Causes Learning Deficits in Mice Post-weaning
Biological Trace Element Research (2021)
-
Indirubin, a small molecular deriving from connectivity map (CMAP) screening, ameliorates obesity-induced metabolic dysfunction by enhancing brown adipose thermogenesis and white adipose browning
Nutrition & Metabolism (2020)