Abstract
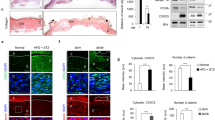
Corneal wounds usually heal quickly; but diabetic patients have more fragile corneas and experience delayed and painful healing. In the present study, we compared the healing capacity of corneal epithelial cells (CECs) between normal and diabetic conditions and the potential mechanisms. Primary murine CEC derived from wild-type and diabetic (db/db) mice, as well as primary human CEC were prepared. Human CEC were exposed to high glucose (30 mM) to mimic diabetic conditions. Cell migration and proliferation were assessed using Scratch test and MTT assays, respectively. Reactive oxygen species (ROS) production in the cells was measured using dichlorofluorescein reagent. Western blot was used to evaluate the expression levels of Akt. Transepithelial electrical resistance (TEER) and zonula occludens-1 (ZO-1) expression were used to determine tight junction integrity. We found that the diabetic CEC displayed significantly slower cell proliferation and migration compared with the normal CEC from both mice and humans. Furthermore, ROS production was markedly increased in CEC grown under diabetic conditions. Treatment with an antioxidant N-acetyl cysteine (NAC, 100 μM) significantly decreased ROS production and increased wound healing in diabetic CEC. Barrier function was significantly reduced in both diabetic mouse and human CEC, while NAC treatment mitigated these effects. We further showed that Akt signaling was impaired in diabetic CEC, which was partially improved by NAC treatment. These results show that diabetic conditions lead to delayed wound-healing capacity of CEC and impaired tight junction formation in both mice and human. Increased ROS production and inhibited Akt signaling may contribute to this outcome, implicating these as potential targets for treating corneal wounds in diabetic patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mendis S, Davis S, Norrving B. Organizational update the World Health Organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46:E121–E2.
Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17:20–33.
Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005;89:254–5.
Abdelkader H, Patel DV, McGhee CNJ, Alany RG. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Exp Ophthalmol. 2011;39:259–70.
Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, et al. Corneal epithelial barrier function in diabetic patients. Cornea. 2004;23:35–7.
Azar DT, Spurrmichaud SJ, Tisdale AS, Gipson IK. Altered epithelial basement-membrane interactions in diabetic corneas. Arch Ophthalmol-Chic. 1992;110:537–40.
Desai BM, Lavingia BC. Cornea thickness and tear glucose levels in diabetes mellitus and normal persons. Indian J Ophthalmol. 1987;35:130–2.
Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Retin Eye Res. 1999;18:293–309.
Yin J, Yu FS. Rho kinases regulate corneal epithelial wound healing. Am J Physiol Cell Physiol. 2008;295:C378–87.
McCartney MD, Cantu-Crouch D. Rabbit corneal epithelial wound repair: tight junction reformation. Curr Eye Res. 1992;11:15–24.
Li J, Zhang R, Wang C, Wang X, Xu M, Ma J, et al. Activation of the small GTPase Rap1 inhibits choroidal neovascularization by regulating cell junctions and ROS generation in rats. Curr Eye Res. 2018;43:934–40.
Yu L, Gan X, Liu X, An R. Calcium oxalate crystals induces tight junction disruption in distal renal tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling pathway. Ren Fail. 2017;39:440–51.
Dehdashtian E, Mehrzadi S, Yousefi B, Hosseinzadeh A, Reiter RJ, Safa M, et al. Diabetic retinopathy pathogenesis and the ameliorating effects of melatonin; involvement of autophagy, inflammation and oxidative stress. Life Sci. 2018;193:20–33.
Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–85.
Zhang Z, Hu X, Qi X, Di G, Zhang Y, Wang Q, et al. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Mol Vis. 2018;24:274–85.
Shi L, Chen HM, Yu XM, Wu XY. Advanced glycation end products delay corneal epithelial wound healing through reactive oxygen species generation. Mol Cell Biochem. 2013;383:253–9.
Huang CC, Liao RF, Wang F, Tang ST. Characteristics of reconstituted tight junctions after corneal epithelial wounds and ultrastructure alterations of corneas in type 2 diabetic rats. Curr Eye Res. 2016;41:783–90.
Yin J, Huang J, Chen C, Gao N, Wang F, Yu FSX. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophth Vis Sci. 2011;52:6589–96.
Lutty GA. Effects of diabetes on the eye. Invest Ophth Vis Sci. 2013; 54:ORSF81-ORSF87.
Zhang YM, Whaley-Connell AT, Sowers JR, Ren J. Autophagy as an emerging target in cardiorenal metabolic disease: from pathophysiology to management. Pharmacol Ther. 2018;191:1–22.
Zagon IS, Klocek MS, Sassani MW, McLaughlin P. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol-Chic. 2007;125:1082–8.
Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol. 1983;96:368–71.
Friend J, Ishii Y, Thoft RA. Corneal epithelial changes in diabetic rats. Ophthalmic Res. 1982;14:269–78.
Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142:207–11.
Basher AWP, Roberts SM. Ocular manifestations of diabetes-mellitus-diabetic cataracts in dogs. Vet Clin N Am-Small. 1995;25:661–76.
Di GH, Du XL, Qi X, Zhao XW, Duan HY, Li SX, et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest Ophth Vis Sci. 2017;58:4344–54.
Yan CX, Gao N, Sun HJ, Yin J, Lee P, Zhou L, et al. Targeting imbalance between IL-1 beta and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am J Pathol. 2016;186:1466–80.
Nagai N, Ito Y. Therapeutic effects of sericin on diabetic keratopathy in Otsuka Long-Evans Tokushima Fatty rats. World J Diabetes. 2013;4:282–9.
Qu JH, Tian L, Zhang XY, Sun XG. Early central and peripheral corneal microstructural changes in type 2 diabetes mellitus patients identified using in vivo confocal microscopy: a case-control study. Medicine 2017; 96:e7960.
Chang PY, Carrel H, Huang JS, Wang IJ, Hou YC, Chen WL, et al. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol. 2006;142:488–90.
Quadrado MJ, Popper M, Morgado AM, Murta JN, Van Best JA. Diabetes and corneal cell densities in humans by in vivo confocal microscopy. Cornea. 2006;25:761–8.
McDermott AM, Kern TS, Murphy CJ. The effect of elevated extracellular glucose on migration, adhesion and proliferation of SV40 transformed human corneal epithelial cells. Curr Eye Res. 1998;17:924–32.
Chikama T, Wakuta M, Liu Y, Nishida T. Deviated mechanism of wound healing in diabetic corneas. Cornea. 2007;26:S75–S81.
Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat). Adv Exp Med Biol. 1988;246:29–31.
Elizondo RA, Yin ZH, Lu XW, Watsky MA. Effect of vitamin D receptor knockout on cornea epithelium wound healing and tight junctions. Invest Ophth Vis Sci. 2014;55:5245–51.
Yi X, Wang Y, Yu FSX. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Invest Ophth Vis Sci. 2000;41:4093–100.
Cho YE, Song BJ. Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress. Redox Biol. 2018;18:266–78.
Lu L, Wang M, Wei X, Li W. 20-HETE inhibition by HET0016 decreases the blood-brain barrier permeability and brain edema after traumatic brain injury. Front Aging Neurosci. 2018;10:207.
Zhang S, An Q, Wang T, Gao S, Zhou G. Autophagy- and MMP-2/9-mediated reduction and redistribution of ZO-1 contribute to hyperglycemia-increased blood-brain barrier permeability during early reperfusion in stroke. Neuroscience. 2018;377:126–37.
Ko JA, Sotani Y, Ibrahim DG, Kiuchi Y. Role of macrophage migration inhibitory factor (MIF) in the effects of oxidative stress on human retinal pigment epithelial cells. Cell Biochem Funct. 2017;35:426–32.
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88.
Gorin Y, Block K. Nox as a target for diabetic complications. Clin Sci. 2013;125:361–82.
Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–8.
Song BB, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–89.
Bhatt MP, Lim YC, Hwang J, Na S, Kim YM, Ha KS. C-peptide prevents hyperglycemia-induced endothelial apoptosis through inhibition of reactive oxygen species-mediated transglutaminase 2 activation. Diabetes. 2013;62:243–53.
Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013;33:2996–3010.
Kim J, Kim CS, Kim H, Jeong IH, Sohn E, Kim JS. Protection against advanced glycation end products and oxidative stress during the development of diabetic keratopathy by KIOM-79. J Pharm Pharmacol. 2011;63:524–30.
Couture C, Desjardins P, Zaniolo K, Germain L, Guerin SL. Enhanced wound healing of tissue-engineered human corneas through altered phosphorylation of the CREB and AKT signal transduction pathways. Acta Biomater. 2018;73:312–25.
Yang LL, Di GH, Qi X, Qu ML, Wang Y, Duan HY, et al. Substance P promotes diabetic corneal epithelial wound healing through molecular mechanisms mediated via the neurokinin-1 receptor. Diabetes. 2014;63:4262–74.
Xu KP, Yu FSX. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophth Vis Sci. 2011;52:3301–8.
Wang S, Zhu X, Xiong L, Ren J. Ablation of Akt2 prevents paraquat-induced myocardial mitochondrial injury and contractile dysfunction: role of Nrf2. Toxicol Lett. 2017;269:1–14.
Priyadarsini S, Rowsey TG, Ma JX, Karamichos D. Unravelling the stromal-nerve interactions in the human diabetic cornea. Exp Eye Res. 2017;164:22–30.
Acknowledgements
This work was supported by grants from the National Institutes of Health (NIH) to HZ (AR067766 and HL124122) and a fellowship from the China Scholarship Council (CSC no. 201706780018) to QJ.
Author contributions
JH, ZS, JM, YL, HC and HZ designed research and provided valuable inputs during the progress of the project; QJ, DK, JF, CL, BG, TT and HC performed research; QJ, YL, HC and HZ analyzed data; QJ, HC and HZ wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Jiang, Qw., Kaili, D., Freeman, J. et al. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol Sin 40, 1205–1211 (2019). https://doi.org/10.1038/s41401-019-0223-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0223-y
Keywords
This article is cited by
-
Topical and oral peroxisome proliferator-activated receptor-α agonist ameliorates diabetic corneal neuropathy
Scientific Reports (2024)
-
Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives
Current Medical Science (2024)
-
Rutin alleviates colon lesions and regulates gut microbiota in diabetic mice
Scientific Reports (2023)