Abstract
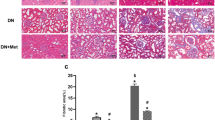
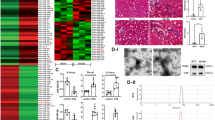
Epithelial–mesenchymal transition (EMT) of renal tubular epithelial cells is one of the potential mechanisms of renal fibrosis, which promotes the development of diabetic nephropathy (DN). However, the molecular mechanisms of EMT remain largely unknown. Tuberous sclerosis proteins TSC1 and TSC2 are key integrators of growth factor signaling, and the loss of TSC1 or TSC2 function leads to a spectrum of diseases that underlie abnormalities in cell growth, proliferation, differentiation, and migration. In this study, we investigated the effects of TSC1 on high glucose (HG)-induced EMT of human proximal tubular epithelial HK-2 cells in vitro and renal fibrosis in TSC1−/− and db/db mice. We found that the exposure of HK-2 cells to HG (30 mM) time-dependently decreased TSC1 expression, increased the phosphorylation of mTORC1, P70S6K, and 4E-BP-1, and promoted cell migration, resulting in EMT. Transfection of the cells with TSC1 mimic significantly ameliorated HG-induced EMT of HK-2 cells. The tubules-specific TSC1 knockout mice (TSC1−/−) displayed a significant decline in renal function. TSC1−/− mice, similar to db/db mice, showed greatly activated mTORC1 signaling and EMT process in the renal cortex and exacerbated renal fibrosis. Overexpression of TSC1 through LV-TSC1 transfection significantly alleviated the progression of EMT and renal fibrosis in the renal cortex of db/db mice. Taken together, our results suggest that TSC1 plays a key role in mediating HG-induced EMT, and inhibition of TSC1-regulated mTORC1 signaling may be a potential approach to prevent renal fibrosis in DN.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
13 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41401-021-00785-3
References
Rossing P. Diabetic nephropathy: worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep. 2006;6:479–83.
Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6:395–423.
Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007;71:846–54.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–84.
Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol. 2010;21:212–22.
Switon K, Kotulska K, Janusz-Kaminska A, Zmorzynska J, Jaworski J. Tuberous sclerosis complex: from molecular biology to novel therapeutic approaches. IUBMB Life. 2016;68:955–62.
Inoki K. Role of TSC-mTOR pathway in diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S59–62.
Hasbani DM, Crino PB. Tuberous sclerosis complex. Handb Clin Neurol. 2018;148:813–22.
Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–57.
Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–93.
Lee CH, Inoki K, Guan KL. mTOR pathway as a target in tissue hypertrophy. Annu Rev Pharm Toxicol. 2007;47:443–67.
Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90.
Wataya-Kaneda M. Mammalian target of rapamycin and tuberous sclerosis complex. J Dermatol Sci. 2015;79:93–100.
Thien A, Prentzell MT, Holzwarth B, Kläsener K, Kuper I, Boehlke C, et al. TSC1 activates TGF-β-Smad2/3 signaling in growth arrest and epithelial-to-mesenchymal transition. Dev Cell. 2015;32:617–30.
Barnes EA, Kenerson HL, Jiang X, Yeung RS. Tuberin regulates E-cadherin localization: implications in epithelial-mesenchymal transition. Am J Pathol. 2010;177:1765–78.
Habib SL, Yadav M, Tizani S, Bhandari B, Valente AJ. Tuberin inhibits production of the matrix protein fibronectin in diabetes. J Am Soc Nephrol. 2012;23:1652–62.
Yao Y, Wang J, Yoshida S, Nada S, Okada M, Inoki K. Role of ragulator in the regulation of mechanistic target of rapamycin signaling in podocytes and glomerular function. J Am Soc Nephrol. 2016;27:3653–65.
Zhang Z, Li BY, Li XL, Cheng M, Yu F, Lu WD, et al. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim Biophys Acta. 2013;1832:805–16.
Lu Q, Ji XJ, Zhou YX, Yao XQ, Liu YQ, Zhang F, et al. Quercetin inhibits the mTORC1/p70S6K signaling-mediated renal tubular epithelial-mesenchymal transition and renal fibrosis in diabetic nephropathy. Pharm Res. 2015;99:237–47.
Kapoor A, Girard L, Lattouf JB, Pei Y, Rendon R, Card P, et al. Evolving strategies in the treatment of Tuberous Sclerosis Complex-associated Angiomyolipomas (TSC-AML). Urology. 2016;89:19–26.
Randle SC. Tuberous sclerosis complex: a review. Pediatr Ann. 2017;46:e166–e171.
Leung AK, Robson WL. Tuberous sclerosis complex: a review. J Pediatr Health Care. 2007;21:108–14.
Ren S, Luo Y, Chen H, Warburton D, Lam HC, Wang LL, et al. Inactivation of Tsc2 in mesoderm-derived cells causes polycystic kidney lesions and impairs lung alveolarization. Am J Pathol. 2016;186:3261–72.
Zhou J, Brugarolas J, Parada LF. Loss of Tsc1, but not Pten, in renal tubular cells causes polycystic kidney disease by activating mTORC1. Hum Mol Genet. 2009;18:4428–41.
Carew RM, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347:103–16.
Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev. 2011;22:131–9.
Tyryshkin A, Bhattacharya A, Eissa NT. SRC kinase is a novel therapeutic target in lymphangioleiomyomatosis. Cancer Res. 2014;74:1996–2005.
Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–72.
Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89.
Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181–96.
Kajiwara M, Masuda S. Role of mTOR inhibitors in kidney disease. Int J Mol Sci. 2016;17:E975.
Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. 2005;16:1384–91.
Lu Q, Zuo WZ, Ji XJ, Zhou YX, Liu YQ, Yao XQ, et al. Ethanolic Ginkgo biloba leaf extract prevents renal fibrosis through Akt/mTOR signaling in diabetic nephropathy. Phytomedicine. 2015;22:1071–8.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 81473257, 81400741), the Qing Lan project, the Natural Science Foundation of Jiangsu Province (No. BK20151155), the “333” Foundation of Jiangsu Province (No. BRA2015329), the Key Natural Science Foundation of Jiangsu Higher Education Institutions of China (No. 15KJA310005), the Jiangsu Overseas Research & Training Program for University Young & Middle-aged Teachers and Presidents, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
XXY designed and supervised the study and revised the paper; QL, YBC, HY, and WWW performed most of the experiments; CCL and LW performed some of the experiments and analyzed part of the data; JW made the figures; LD purchased the reagents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Lu, Q., Chen, Yb., Yang, H. et al. Inactivation of TSC1 promotes epithelial-mesenchymal transition of renal tubular epithelial cells in mouse diabetic nephropathy. Acta Pharmacol Sin 40, 1555–1567 (2019). https://doi.org/10.1038/s41401-019-0244-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0244-6
Keywords
This article is cited by
-
LncRNA GAS5 reduces blood glucose levels and alleviates renal fibrosis in diabetic nephropathy by regulating the miR-542-3p/ERBB4 axis
Diabetology & Metabolic Syndrome (2025)
-
The role of the farnesoid X receptor in diabetes and its complications
Molecular and Cellular Biochemistry (2025)
-
P4HB regulates the TGFβ/SMAD3 signaling pathway through PRMT1 to participate in high glucose-induced epithelial-mesenchymal transition and fibrosis of renal tubular epithelial cells
BMC Nephrology (2024)
-
YY1 inactivated transcription co-regulator PGC-1α to promote mitochondrial dysfunction of early diabetic nephropathy-associated tubulointerstitial fibrosis
Cell Biology and Toxicology (2023)
-
HG-Induced sEVs Mediate Biomechanics of HK-2 Cells
Nanomanufacturing and Metrology (2023)