Abstract
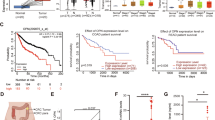
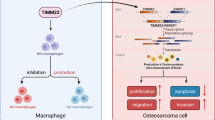
Emerging evidence indicates that M2-polarized tumor-associated macrophages (TAMs) directly participate in tumor initiation, progression and metastasis. However, to date, few studies have investigated novel strategies for inhibiting TAMs in order to overcome osteosarcoma. In this study, we reported that M2 macrophages were enriched in osteosarcoma tissues from patients, and M2-polarized TAMs enhanced cancer initiation and stemness of osteosarcoma cells, thereby establishing M2-polarized TAMs as a therapeutic target for blocking osteosarcoma formation. We also found that all-trans retinoic acid (ATRA) weakened TAM-induced osteosarcoma tumor formation by inhibiting M2 polarization of TAMs in vivo, and inhibited the colony formation, as well as sphere-formation capacity of osteosarcoma cells promoted by M2-type macrophages in vitro. Furthermore, M2-type macrophages enhanced cancer stem cells (CSCs) properties as assessed by increasing the numbers of CD117+Stro-1+ cells accompanied by the upregulation of CSC markers (CD133, CXCR4, Nanog, and Oct4), which could clearly be reduced by ATRA. Taken together, the results of this study demonstrated the role of M2-polarized TAMs in osteosarcoma initiation and stemness by activating CSCs, and indicated that ATRA treatment is a promising approach for treating osteosarcoma by preventing M2 polarization of TAMs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35.
Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma—connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–91.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73.
Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–62.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–82.
Bae JH, Park SH, Yang JH, Yang K, Yi JM. Stem cell-like gene expression signature identified in ionizing radiation-treated cancer cells. Gene. 2015;572:285–91.
Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 2011;121:3804–9.
Fessler E, Dijkgraaf FE, De Sousa EMF, Medema JP. Cancer stem cell dynamics in tumor progression and metastasis: is the microenvironment to blame? Cancer Lett. 2013;341:97–104.
Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–90.
Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, et al. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–46.
Yu G, Jing Y, Kou X, Ye F, Gao L, Fan Q, et al. Hepatic stellate cells secreted hepatocyte growth factor contributes to the chemoresistance of hepatocellular carcinoma. PLoS ONE. 2013;8:e73312.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Raggi C, Mousa HS, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35:671–82.
Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–8.
Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells. 2012;31:248–58.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA. 2011;108:12425–30.
Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41.
Nusblat LM, Carroll MJ, Roth CM. Crosstalk between M2 macrophages and glioma stem cells. Cell Oncol (Dordr). 2017;40:471–82.
Zhou Q, Xian M, Xiang S, Xiang D, Shao X, Wang J, et al. All-trans retinoic acid prevents osteosarcoma metastasis by inhibiting M2 polarization of tumor-associated macrophages. Cancer Immunol Res. 2017;5:547–59.
Fan TM. Animal models of osteosarcoma. Expert Rev Anticancer Ther. 2010;10:1327–38.
Yao Z, Zhang J, Zhang B, Liang G, Chen X, Yao F, et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. Pharm Res. 2018;133:121–31.
Tariq M, Zhang JQ, Liang GK, He QJ, Ding L, Yang B. Gefitinib inhibits M2-like polarization of tumor-associated macrophages in Lewis lung cancer by targeting the STAT6 signaling pathway. Acta Pharmacol Sin. 2017;38:1501–11.
Xu T, Huang C, Qi XT, Yang XC, Zhang N, Cao J, et al. 2-Bromopalmitate sensitizes osteosarcoma cells to adriamycin-induced apoptosis via the modulation of CHOP. Eur J Pharmacol. 2018;844:204–15.
Qi XT, Li YL, Zhang YQ, Xu T, Lu B, Fang L, et al. KLF4 functions as an oncogene in promoting cancer stem cell-like characteristics in osteosarcoma cells. Acta Pharmacol Sin. 2019;40:546–55.
Gatti M, Solari A, Pattarozzi A, Campanella C, Thellung S, Maniscalco L, et al. In vitro and in vivo characterization of stem-like cells from canine osteosarcoma and assessment of drug sensitivity. Exp Cell Res. 2018;363:48–64.
Huynh DL, Kwon T, Zhang JJ, Sharma N, Gera M, Ghosh M, et al. Wogonin suppresses stem cell-like traits of CD133 positive osteosarcoma cell via inhibiting matrix metallopeptidase-9 expression. BMC Complement Alter Med. 2017;17:304.
Paiva-Oliveira DI, Martins-Neves SR, Abrunhosa AJ, Fontes-Ribeiro C, Gomes CMF. Therapeutic potential of the metabolic modulator metformin on osteosarcoma cancer stem-like cells. Cancer Chemother Pharmacol. 2018;81:49–63.
Moran EM. Epidemiological and clinical aspects of nonsteroidal anti-inflammatory drugs and cancer risks. J Environ Pathol Toxicol Oncol. 2002;21:193–201.
Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45.
Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41.
Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014;35:1780–7.
Young MJ, Wu YH, Chiu WT, Weng TY, Huang YF, Chou CY. All-trans retinoic acid downregulates ALDH1-mediated stemness and inhibits tumour formation in ovarian cancer cells. Carcinogenesis. 2015;36:498–507.
Nguyen PH, Giraud J, Staedel C, Chambonnier L, Dubus P, Chevret E, et al. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene. 2016;35:5619–28.
Ying M, Zhang L, Zhou Q, Shao X, Cao J, Zhang N, et al. The E3 ubiquitin protein ligase MDM2 dictates all-trans retinoic acid-induced osteoblastic differentiation of osteosarcoma cells by modulating the degradation of RARalpha. Oncogene. 2016;35:4358–67.
Yang QJ, Zhou LY, Mu YQ, Zhou QX, Luo JY, Cheng L, et al. All-trans retinoic acid inhibits tumor growth of human osteosarcoma by activating Smad signaling-induced osteogenic differentiation. Int J Oncol. 2012;41:153–60.
Acknowledgements
This work was supported by the State Key Program of the National Natural Science Foundation of China (81830107 to QJH) and by grants from the National Natural Science Foundation of China (81473225 to QJH, 81803552 to XJS, and 81603126 to NZ).
Author information
Authors and Affiliations
Contributions
QJH, MDY, QZ, and XJS designed the research project; QZ, XJS, SFX, and YQC performed the experiments; QJH, MDY, QZ, XJS, JC, HZ, and BY analyzed the data; NZ contributed patient samples; and QJH, MDY, QZ, and XJS wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Shao, Xj., Xiang, Sf., Chen, Yq. et al. Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells. Acta Pharmacol Sin 40, 1343–1350 (2019). https://doi.org/10.1038/s41401-019-0262-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0262-4
Keywords
This article is cited by
-
Comprehensive analysis of ESCRT transcriptome-associated signatures and identification of the regulatory role of LMO7-AS1 in osteosarcoma
Cancer Cell International (2025)
-
Dynamic assessment of a humanized bone tumour microenvironment reveals insights into osteosarcoma primary tumour remodelling and lung metastases
Scientific Reports (2025)
-
Dual roles and therapeutic targeting of tumor-associated macrophages in tumor microenvironments
Signal Transduction and Targeted Therapy (2025)
-
Multi-omics and Single Cell Sequencing Analyses Reveal Associations of Mitophagy-Related Genes Predicting Clinical Prognosis and Immune Infiltration Characteristics in Osteosarcoma
Molecular Biotechnology (2025)
-
Advances on immunotherapy for osteosarcoma
Molecular Cancer (2024)