Abstract
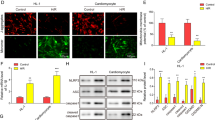
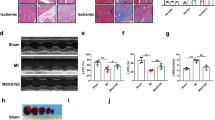
Pyroptosis is a form of inflammatory cell death that could be driven by the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation following myocardial infarction (MI). Emerging evidence suggests the therapeutic potential for ameliorating MI-induced myocardial damages by targeting NLRP3 and pyroptosis. In this study, we investigated the myocardial protection effect of a novel anthraquinone compound (4,5-dihydroxy-7-methyl-9,10-anthraquinone-2-ethyl succinate) named Kanglexin (KLX) in vivo and in vitro. Male C57BL/6 mice were pre-treated either with KLX (20, 40 mg· kg−1per day, intragastric gavage) or vehicle for 7 consecutive days prior to ligation of coronary artery to induce permanent MI. KLX administration dose-dependently reduced myocardial infarct size and lactate dehydrogenase release and improved cardiac function as compared to vehicle-treated mice 24 h after MI. We found that MI triggered NLRP3 inflammasome activation leading to conversion of interleukin-1β (IL-1β) and IL-18 into their active mature forms in the heart, which could expand the infarct size and drive cardiac dysfunction. We also showed that MI induced pyroptosis, as evidenced by increased DNA fragmentation, mitochondrial swelling, and cell membrane rupture, as well as increased levels of pyroptosis-related proteins, including gasdermin D, N-terminal GSDMD, and cleaved caspase-1. All these detrimental alterations were prevented by KLX. In hypoxia- or lipopolysaccharide (LPS)-treated neonatal mouse ventricular cardiomyocytes, we showed that KLX (10 μM) decreased the elevated levels of terminal deoxynucleotidyl transferase dUTP nick end labeling- and propidium iodide-positive cells, and pyroptosis-related proteins. We conclude that KLX prevents MI-induced cardiac damages and cardiac dysfunction at least partly through attenuating NLRP3 and subsequent cardiomyocyte pyroptosis, and it is worthy of more rigorous investigations for its potential for alleviating ischemic heart disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–82.
Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619.
Wang PP, Zhang YJ, Xie T, Sun J, Wang XD. MiR-223 promotes cardiomyocyte apoptosis by inhibiting Foxo3a expression. Eur Rev Med Pharmacol Sci. 2018;22:6119–26.
Olivier E, Dutot M, Regazzetti A, Laprevote O, Rat P. 25-Hydroxycholesterol induces both P2X7-dependent pyroptosis and caspase-dependent apoptosis in human skin model: New insights into degenerative pathways. Chem Phys Lipids. 2017;207:171–8.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109.
Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA. 2011;108:19725–30.
Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203–14.
Takahashi M. NLRP3 inflammasome as a novel player in myocardial infarction. Int Heart J. 2014;55:101–5.
Lei Q, Yi T, Chen C. NF-kappaB-Gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–52.
Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O’Donnell K, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113:32.
Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun A, et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun. 2018;499:267–72.
Yu Y, Liu H, Yang D, He F, Yuan Y, Guo J, et al. Aloe-Emodin attenuates myocardial infarction and apoptosis via up-regulating MiR-133 expression. Pharmacol Res. 2019;146:104315.
Huang J, Li X, Liu P, Wang J, Li H. Emodin protects H9c2 cells against hypoxia-induced injury via regulation of miR-26a/survivin and the JAK1/STAT3 pathway. J Cell Biochem. 2019; https://doi.org/10.1002/jcb.28385. (In press).
Yang Y, Jiang Z, Zhuge D. Emodin attenuates lipopolysaccharide-induced injury via down-regulation of miR-223 in H9c2 cells. Int Heart J. 2019;60:436–43.
Liu J, Li Y, Tang Y, Cheng J, Wang J, Li J, et al. Rhein protects the myocardiac cells against hypoxia/reoxygention-induced injury by suppressing GSK3beta activity. Phytomedicine. 2018;51:1–6.
Bai Y, Su Z, Sun H, Zhao W, Chen X, Hang P, et al. Aloe-emodin relieves high-fat diet induced QT prolongation via MiR-1 inhibition and IK1 up-regulation in rats. Cell Physiol Biochem. 2017;43:1961–73.
Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–72.
Mezzaroma E, Toldo S, Abbate A. Role of NLRP3 (cryopyrin) in acute myocardial infarction. Cardiovasc Res. 2013;99:225–6.
Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia–reperfusion in the mouse. Int J Cardiol. 2016;209:215–20.
Zhang Y, Jiao L, Sun L, Li Y, Gao Y, Xu C, et al. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res. 2018;122:1354–68.
Zhou Z, Wang Z, Guan Q, Qiu F, Li Y, Liu Z, et al. PEDF inhibits the activation of NLRP3 inflammasome in hypoxia cardiomyocytes through PEDF receptor/phospholipase A2. Int J Mol Sci. 2016;17, pii: E2064.
Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54.
Chen J, Wang B, Lai J, Braunstein Z, He M, Ruan G, et al. Trimetazidine attenuates cardiac dysfunction in endotoxemia and sepsis by promoting neutrophil migration. Front Immunol. 2018;9:2015.
Arnold SV, Spertus JA, Masoudi FA, Daugherty SL, Maddox TM, Li Y, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–801.
Love JN, Enlow B, Howell JM, Klein-Schwartz W, Litovitz TL. Electrocardiographic changes associated with beta-blocker toxicity. Ann Emerg Med. 2002;40:603–10.
Boggild M. Intracerebral haemorrhage after dermal nitrate application. BMJ. 1992;305:1000.
Wu B, Lin J. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. Biomed Res Int. 2017;2017:3764370.
Pan C, Lou L, Huo Y, Singh G, Chen M, Zhang D, et al. Salvianolic acid B and tanshinone IIA attenuate myocardial ischemia injury in mice by NO production through multiple pathways. Ther Adv Cardiovasc Dis. 2011;5:99–111.
Nazir S, Gadi I, Al-Dabet MM, Elwakiel A, Kohli S, Ghosh S, et al. Cytoprotective activated protein C averts Nlrp3 inflammasome-induced ischemia–reperfusion injury via mTORC1 inhibition. Blood. 2017;130:2664–77.
Dolunay A, Senol SP, Temiz-Resitoglu M, Guden DS, Sari AN, Sahan-Firat S, et al. Inhibition of NLRP3 inflammasome prevents LPS-induced inflammatory hyperalgesia in mice: contribution of NF-kappaB, caspase-1/11, ASC, NOX, and NOS isoforms. Inflammation. 2017;40:366–86.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20.
van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38:828–36.
Bullon P, Cano-Garcia FJ, Alcocer-Gomez E, Varela-Lopez A, Roman-Malo L, Ruiz-Salmeron RJ, et al. Could NLRP3-inflammasome be a cardiovascular risk biomarker in acute myocardial infarction patients? Antioxid Redox Signal. 2017;27:269–75.
Deten A, Volz HC, Briest W, Zimmer HG. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res. 2002;55:329–40.
Abbate A, Van Tassell BW, Seropian IM, Toldo S, Robati R, Varma A, et al. Interleukin-1beta modulation using a genetically engineered antibody prevents adverse cardiac remodelling following acute myocardial infarction in the mouse. Eur J Heart Fail. 2010;12:319–22.
Venkatachalam K, Prabhu SD, Reddy VS, Boylston WH, Valente AJ, Chandrasekar B. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J Biol Chem. 2009;284:7853–65.
Jong WM, Leemans JC, Weber NC, Juffermans NP, Schultz MJ, Hollmann MW, et al. Nlrp3 plays no role in acute cardiac infarction due to low cardiac expression. Int J Cardiol. 2014;177:41–3.
Yin J, Wang Y, Hu H, Li X, Xue M, Cheng W, et al. P2X7 receptor inhibition attenuated sympathetic nerve sprouting after myocardial infarction via the NLRP3/IL-1beta pathway. J Cell Mol Med. 2017;21:2695–710.
Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, De Kleijn DPV, et al. Innate immunity as a target for acute cardioprotection. Cardiovasc Res. 2019;115:1131–42.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (grants number: 81730012, 81603105, 81773733, and 81573425), the Scientific Research Starting Foundation for Returned Overseas Chinese Scholars of Heilongjiang Province, the Heilongjiang Natural Science Foundation (grants number: H2016010), and the China Postdoctoral Science Foundation (2017M621309).
Author information
Authors and Affiliations
Contributions
BFY, WX, WJD, NW, and YB designed the present study; YB, XL, PP, XLH, STY, and YNL performed the experiments; JHW conducted the synthesis of KLX; YB, XL, PP, and XL analyzed the data; YB, WJD, and BFY wrote the paper; WJD and NW revised paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Bian, Y., Li, X., Pang, P. et al. Kanglexin, a novel anthraquinone compound, protects against myocardial ischemic injury in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacol Sin 41, 319–326 (2020). https://doi.org/10.1038/s41401-019-0307-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0307-8
Keywords
This article is cited by
-
Circulating extracellular vesicles regulate ELAVL1 by delivering miR-133a-3p which affecting NLRP3 mRNA stability inhibiting PANoptosome formation
Biology Direct (2025)
-
IFI204 in microglia mediates traumatic brain injury-induced mitochondrial dysfunction and pyroptosis via SENP7 interaction
Cell Biology and Toxicology (2025)
-
A novel deacetylase inhibitor KLX suppresses liver fibrosis by deacetylating PPARγ through promoting ubiquitination-mediated HDAC1 degradation
Science China Life Sciences (2025)
-
Ginsenoside Rg1 mitigates myocardial ischemia/reperfusion injury by inhibiting NLRP3-mediated pyroptosis
In Vitro Cellular & Developmental Biology - Animal (2025)
-
KLX ameliorates liver cancer progression by mediating ZBP1 transcription and ubiquitination and increasing ZBP1-induced PANoptosis
Acta Pharmacologica Sinica (2025)