Abstract
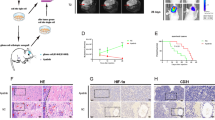
Xanthatin is a natural sesquiterpene lactone purified from Xanthium strumarium L., which has shown prominent antitumor activity against a variety of cancer cells. In the current study, we investigated the effect of xanthatin on the growth of glioma cells in vitro and in vivo, and elucidated the underlying mechanisms. In both rat glioma C6 and human glioma U251 cell lines, xanthatin (1–15 μM) dose-dependently inhibited cell viability without apparent effect on the cell cycle. Furthermore, xanthatin treatment dose-dependently induced glioma cell apoptosis. In nude mice bearing C6 glioma tumor xenografts, administration of xanthatin (10, 20, 40 mg·kg−1·d−1, ip, for 2 weeks) dose-dependently inhibited the tumor growth, but did not affect the body weight. More importantly, xanthatin treatment markedly increased the expression levels of the endoplasmic reticulum (ER) stress-related markers in both the glioma cell lines as well as in C6 xenografts, including glucose-regulated protein 78, C/EBP-homologous protein (CHOP), activating factor 4, activating transcription factor 6, spliced X-box binding protein-1, phosphorylated protein kinase R-like endoplasmic reticulum kinase, and phosphorylated eukaryotic initiation factor 2a. Pretreatment of C6 glioma cells with the ER stress inhibitor 4-phenylbutyric acid (4-PBA, 7 mM) or knockdown of CHOP using small interfering RNA significantly attenuated xanthatin-induced cell apoptosis and increase of proapoptotic caspase-3. These results demonstrate that xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating the ER stress-related unfolded protein response pathway involving CHOP induction. Xanthatin may serve as a promising agent in the treatment of human glioma.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Szopa W, Burley TA, Kramer-Marek G, Kaspera W. Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. Biomed Res Int. 2017;2017:8013575.
Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–46.
Ghotme KA, Barreto GE, Echeverria V, Gonzalez J, Bustos RH, Sanchez M, et al. Gliomas: new perspectives in diagnosis, treatment and prognosis. Curr Top Med Chem. 2017;17:1438–47.
Lee BH, Yoon SH, Kim YS, Kim SK, Moon BJ, Bae YS. Apoptotic cell death through inhibition of protein kinase CKII activity by 3,4-dihydroxybenzaldehyde purified from Xanthium strumarium. Nat Prod Res. 2008;22:1441–50.
Aranjani JM, Manuel A, Mallikarjuna Rao C, Udupa N, Rao JV, Joy AM, et al. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of Xanthium strumarium in transplantable tumors in mice. Am J Chin Med. 2013;41:145–62.
Liu R, Shi D, Zhang J, Li X, Han X, Yao X, et al. Xanthatin promotes apoptosis via inhibiting thioredoxin reductase and eliciting oxidative stress. Mol Pharm. 2018;15:3285–96.
Ramirez-Erosa I, Huang Y, Hickie RA, Sutherland RG, Barl B. Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can J Physiol Pharmacol. 2007;85:1160–72.
Shen M, Zhou XZ, Ye L, Yuan Q, Shi C, Zhu PW, et al. Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2 mediated STAT3/PI3K/Akt signaling pathway. Int J Mol Med. 2018;42:769–78.
Yu Y, Yu J, Pei CG, Li YY, Tu P, Gao GP, et al. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int J Clin Exp Pathol. 2015;8:10355–64.
Romero M, Zanuy M, Rosell E, Cascante M, Piulats J, Font-Bardia M, et al. Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties. Eur J Med Chem. 2015;90:491–6.
Nibret E, Youns M, Krauth-Siegel RL, Wink M. Biological activities of xanthatin from Xanthium strumarium leaves. Phytother Res. 2011;25:1883–90.
Pinel B, Landreau A, Seraphin D, Larcher G, Bouchara JP, Richomme P. Synthesis of reduced xanthatin derivatives and in vitro evaluation of their antifungal activity. J Enzym Inhib Med Chem. 2005;20:575–9.
Lavault M, Landreau A, Larcher G, Bouchara JP, Pagniez F, Le Pape P, et al. Antileishmanial and antifungal activities of xanthanolides isolated from Xanthium macrocarpum. Fitoterapia. 2005;76:363–6.
Sato Y, Oketani H, Yamada T, Singyouchi K, Ohtsubo T, Kihara M, et al. A xanthanolide with potent antibacterial activity against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol. 1997;49:1042–4.
Tao L, Cao Y, Wei Z, Jia Q, Yu S, Zhong J, et al. Xanthatin triggers Chk1-mediated DNA damage response and destabilizes Cdc25C via lysosomal degradation in lung cancer cells. Toxicol Appl Pharmacol. 2017;337:85–94.
Tao L, Fan F, Liu Y, Li W, Zhang L, Ruan J, et al. Concerted suppression of STAT3 and GSK3beta is involved in growth inhibition of non-small cell lung cancer by xanthatin. PLoS One. 2013;8:e81945.
Takeda S, Noguchi M, Matsuo K, Yamaguchi Y, Kudo T, Nishimura H, et al. (-)-Xanthatin up-regulation of the GADD45gamma tumor suppressor gene in MDA-MB-231 breast cancer cells: role of topoisomerase IIalpha inhibition and reactive oxygen species. Toxicology. 2013;305:1–9.
Takeda S, Nishimura H, Koyachi K, Matsumoto K, Yoshida K, Okamoto Y, et al. (-)-Xanthatin induces the prolonged expression of c-Fos through an N-acetyl-L-cysteine (NAC)-sensitive mechanism in human breast cancer MDA-MB-231 cells. J Toxicol Sci. 2013;38:547–57.
Li WD, Wu Y, Zhang L, Yan LG, Yin FZ, Ruan JS, et al. Characterization of xanthatin: anticancer properties and mechanisms of inhibited murine melanoma in vitro and in vivo. Phytomedicine. 2013;20:865–73.
Zhang L, Ruan J, Yan L, Li W, Wu Y, Tao L, et al. Xanthatin induces cell cycle arrest at G2/M checkpoint and apoptosis via disrupting NF-kappaB pathway in A549 non-small cell lung cancer cells. Molecules. 2012;17:3736–50.
Takeda S, Matsuo K, Yaji K, Okajima-Miyazaki S, Harada M, Miyoshi H, et al. (-)-Xanthatin selectively induces GADD45gamma and stimulates caspase-independent cell death in human breast cancer MDA-MB-231 cells. Chem Res Toxicol. 2011;24:855–65.
Zhang L, Tao L, Ruan J, Li W, Wu Y, Yan L, et al. Xanthatin induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma MKN-45 cells. Planta Med. 2012;78:890–5.
Tao L, Sheng X, Zhang L, Li W, Wei Z, Zhu P, et al. Xanthatin anti-tumor cytotoxicity is mediated via glycogen synthase kinase-3beta and beta-catenin. Biochem Pharmacol. 2016;115:18–27.
Bi SX, Li XH, Wei CS, Xiang HH, Shen YX, Yu YQ. The antitumour growth and antiangiogenesis effects of xanthatin in murine glioma dynamically evaluated by dynamic contrast-enhanced magnetic resonance imaging. Phytother Res. 2019;33:149–58.
Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–90.
Bettigole SE, Glimcher LH. Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 2015;33:107–38.
Johnson GG, White MC, Grimaldi M. Stressed to death: targeting endoplasmic reticulum stress response induced apoptosis in gliomas. Curr Pharm Des. 2011;17:284–92.
Obacz J, Avril T, Le Reste PJ, Urra H, Quillien V, Hetz C, et al. Endoplasmic reticulum proteostasis in glioblastoma-From molecular mechanisms to therapeutic perspectives. Sci Signal. 2017;10:pii: eaal2323.
Le Reste PJ, Avril T, Quillien V, Morandi X, Chevet E. Signaling the unfolded protein response in primary brain cancers. Brain Res. 2016;1642:59–69.
Penaranda Fajardo NM, Meijer C, Kruyt FA. The endoplasmic reticulum stress/unfolded protein response in gliomagenesis, tumor progression and as a therapeutic target in glioblastoma. Biochem Pharmacol. 2016;118:1–8.
Shen Y, Sun A, Wang Y, Cha D, Wang H, Wang F, et al. Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J Neuroinflammation. 2012;9:254.
Yu YQ, Liu LC, Wang FC, Liang Y, Cha DQ, Zhang JJ, et al. Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J Cereb Blood Flow Metab. 2010;30:79–91.
Chen L, Feng L, Wang X, Du J, Chen Y, Yang W, et al. Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway. Sci Rep. 2015;5:8133.
Feng L, Zhang J, Zhu N, Ding Q, Zhang X, Yu J, et al. Ubiquitin ligase SYVN1/HRD1 facilitates degradation of the SERPINA1 Z variant/alpha-1-antitrypsin Z variant via SQSTM1/p62-dependent selective autophagy. Autophagy. 2017;13:686–702.
Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52.
Mizukami T, Orihashi K, Herlambang B, Takahashi S, Hamaishi M, Okada K, et al. Sodium 4-phenylbutyrate protects against spinal cord ischemia by inhibition of endoplasmic reticulum stress. J Vasc Surg. 2010;52:1580–6.
Fang XY, Zhang H, Zhao L, Tan S, Ren QC, Wang L, et al. A new xanthatin analogue 1beta-hydroxyl-5alpha-chloro-8-epi-xanthatin induces apoptosis through ROS-mediated ERK/p38 MAPK activation and JAK2/STAT3 inhibition in human hepatocellular carcinoma. Biochimie. 2018;152:43–52.
Babaei G, Aliarab A, Abroon S, Rasmi Y, Aziz SG. Application of sesquiterpene lactone: a new promising way for cancer therapy based on anticancer activity. Biomed Pharmacother. 2018;106:239–46.
Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–97.
Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One. 2009;4:e4170.
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–49.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (81673438 to YXS, 81302755 and 81973337 to LJF), the State’s Key Project of Research and Development Plan (YFC1305902 to YXS), the Key Projects of Anhui Provincial Natural Science Research in Colleges and Universities (KJ2017A167 to LJF), and the Scientific Research Foundation of the Institute for Translational Medicine of Anhui Province (2017zhyx34 to YXS and YQY).
Author information
Authors and Affiliations
Contributions
LJF, YXS, and YQY designed the research; YYM, ZMD, QC, WSX and SXB performed the research; YYM, ZMD, JSY, and YJS analyzed the data; and LJF and YXS wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ma, Yy., Di, Zm., Cao, Q. et al. Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacol Sin 41, 404–414 (2020). https://doi.org/10.1038/s41401-019-0318-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0318-5
Keywords
This article is cited by
-
Phytochemical and pharmacological properties of Xanthium species: a review
Phytochemistry Reviews (2025)
-
Interplay between endoplasmic reticulum stress and non-coding RNAs in cancer
Journal of Hematology & Oncology (2020)