Abstract
Norditerpenoids and dinorditerpenoids represent diterpenoids widely distributed in the genus Podocarpus with notable chemical structures and biological activities. We previously reported that nagilactone E (NLE), a dinorditerpenoid isolated from Podocarpus nagi, possessed anticancer effects against lung cancer cells in vitro. In this study we investigated the in vivo effect of NLE against lung cancer as well as the underlying mechanisms. We administered NLE (10 mg·kg−1·d−1, ip) to CB-17/SCID mice bearing human lung cancer cell line A549 xenograft for 3 weeks. We found that NLE administration significantly suppressed the tumor growth without obvious adverse effects. Thereafter, RNA sequencing (RNA-seq) analysis was performed to study the mechanisms of NLE. The effects of NLE on A549 cells have been illustrated by GO and pathway enrichment analyses. CMap dataset analysis supported NLE to be a potential protein synthesis inhibitor. The inhibitory effect of NLE on synthesis of total de novo protein was confirmed in Click-iT assay. Using the pcDNA3-RLUC-POLIRES-FLUC luciferase assay we further demonstrated that NLE inhibited both cap-dependent and cap-independent translation. Finally, molecular docking revealed the low-energy binding conformations of NLE and its potential target RIOK2. In conclusion, NLE is a protein synthesis inhibitor with anticancer activity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Feng ZL, Zhang LL, Zheng YD, Liu QY, Liu JX, Feng L, et al. Norditerpenoids and dinorditerpenoids from the seeds of Podocarpus nagi as cytotoxic agents and autophagy inducers. J Nat Prod. 2017;80:2110–7.
Addo EM, Chai H-B, Hymete A, Yeshak MY, Slebodnick C, Kingston DG, et al. Antiproliferative constituents of the roots of Ethiopian Podocarpus falcatus and structure revision of 2α-hydroxynagilactone F and nagilactone I. J Nat Prod. 2015;78:827–35.
Hayashi K, Yamaguchi Y, Ogita A, Tanaka T, Kubo I, Fujita KI. Effect of nagilactone E on cell morphology and glucan biosynthesis in budding yeast Saccharomyces cerevisiae. Fitoterapia. 2018;128:112–7.
Feng ZL, Zhang T, Liu JX, Chen XP, Gan LS, Ye Y, et al. New podolactones from the seeds of Podocarpus nagi and their anti-inflammatory effect. J Nat Med. 2018;72:882–9.
Gui Y, Yao S, Yan H, Hu L, Yu C, Gao F, et al. A novel small molecule liver X receptor transcriptional regulator, nagilactone B, suppresses atherosclerosis in apoE-deficient mice. Cardiovasc Res. 2016;112:502–14.
Zhang LL, Feng ZL, Su MX, Jiang XM, Chen X, Wang Y, et al. Downregulation of Cyclin B1 mediates nagilactone E-induced G2 phase cell cycle arrest in non-small cell lung cancer cells. Eur J Pharmacol. 2018;830:17–25.
Zhang LL, Jiang XM, Huang MY, Feng ZL, Chen X, Wang Y, et al. Nagilactone E suppresses TGF-β1-induced epithelial–mesenchymal transition, migration and invasion in non-small cell lung cancer cells. Phytomedicine. 2019;52:32–9.
Luo F, Gu J, Chen L, Xu X. Systems pharmacology strategies for anticancer drug discovery based on natural products. Mol Biosyst. 2014;10:1912–7.
Schenone M, Dančík V, Wagner BK, Clemons PA. Target identification and mechanism of action in chemical biology and drug discovery. Nat Chem Biol. 2013;9:232.
Van Dijk EL, Auger H, Jaszczyszyn Y, Thermes C. Ten years of next-generation sequencing technology. Trends Genet. 2014;30:418–26.
Buermans H, Den Dunnen J. Next generation sequencing technology: advances and applications. BBA-Mol Basis Dis. 2014;1842:1932–41.
Costa V, Angelini C, De Feis I, Ciccodicola A. Uncovering the complexity of transcriptomes with RNA-Seq. BioMed Res Int. 2010;2010:853916.
Wacker SA, Houghtaling BR, Elemento O, Kapoor TM. Using transcriptome sequencing to identify mechanisms of drug action and resistance. Nat Chem Biol. 2012;8:235.
Zhang W, Bouchard G, Yu A, Shafiq M, Jamali M, Shrager JB, et al. GFPT2-expressing cancer-associated fibroblasts mediate metabolic reprogramming in human lung adenocarcinoma. Cancer Res. 2018;78:3445–57.
Poulin F, Gingras AC, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J Biol Chem. 1998;273:14002–7.
Zhang LL, Xu YL, Tang ZH, Xu XH, Chen X, Li T, et al. Effects of alisol B 23-acetate on ovarian cancer cells: G1 phase cell cycle arrest, apoptosis, migration and invasion inhibition. Phytomedicine. 2016;23:800–9.
Abagyan R, Totrov M, Kuznetsov D. ICM—a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15:488–506.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006;313:1929–35.
Michnick SW. The connectivity map. Nat Chem Biol. 2006;2:663.
Lamb J. The Connectivity Map: a new tool for biomedical research. Nat Rev Cancer. 2007;7:54–60.
Vartanian S, Ma TP, Lee J, Haverty PM, Kirkpatrick DS, Yu K, et al. Application of mass spectrometry profiling to establish brusatol as an inhibitor of global protein synthesis. Mol Cell Proteom. 2016;15:1220–31.
Song W, Wang Y, Yu Z, Vera CIR, Qu J, Lin Q. A metabolic alkene reporter for spatiotemporally controlled imaging of newly synthesized proteins in mammalian cells. ACS Chem Biol. 2010;5:875–85.
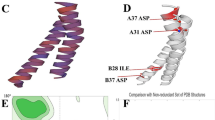
Wang J, Varin T, Vieth M, Elkins JM. Crystal structure of human RIOK2 bound to a specific inhibitor. Open Biol. 2019;9:190037.
Huang MY, Zhang LL, Ding J, Lu JJ. Anticancer drug discovery from Chinese medicinal herbs. Chin Med. 2018;13:35.
Jimenez A, Carrasco L, Vazquez D. Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors. Biochemistry. 1977;16:4727–30.
Gupta RS, Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977;10:61–6.
Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, et al. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov. 2018;4:31. https://doi.org/10.1038/s41421-018-0034-1.
Ennis H, Lubin M. Cycloheximide: aspects of inhibition of protein synthesis in mammalian cells. Science. 1964;146:1474–6.
Harder B, Tian W, La Clair JJ, Tan AC, Ooi A, Chapman E, et al. Brusatol overcomes chemoresistance through inhibition of protein translation. Mol Carcinog. 2017;56:1493–500.
Chan J, Khan SN, Harvey I, Merrick W, Pelletier J. Eukaryotic protein synthesis inhibitors identified by comparison of cytotoxicity profiles. RNA. 2004;10:528–43.
Liu K, Chen HL, Wang S, Gu MM, Chen XM, Zhang SL, et al. High expression of RIOK2 and NOB1 predict human non-small cell lung cancer outcomes. Sci Rep. 2016;6:28666. https://doi.org/10.1038/srep28666.
Acknowledgements
This work was supported by the Science and Technology Development Fund, Macau SAR (File no. 176/2017/A3), and the Research Fund of the University of Macau (MYRG2018-00165-ICMS, CPG2019-00006-ICMS, and MYRG2015-00153-ICMS-QRCM). We thank Prof. Yun Tang (East China University of Science and Technology) for his kindly help with this work.
Author information
Authors and Affiliations
Contributions
LLZ and JJL designed this study. LGL provided the compound NLE. LLZ and JG performed the experiments. LLZ, HL and JJL drafted the paper. LLZ, XMJ, HL and JJL analyzed the data. All the authors participated in the revision and improvement of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, Ll., Guo, J., Jiang, Xm. et al. Identification of nagilactone E as a protein synthesis inhibitor with anticancer activity. Acta Pharmacol Sin 41, 698–705 (2020). https://doi.org/10.1038/s41401-019-0332-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-019-0332-7
Keywords
This article is cited by
-
Expanding the phenotypic and genetic spectrum of GTPBP3 deficiency: findings from nine Chinese pedigrees
Orphanet Journal of Rare Diseases (2024)
-
Berberine remodels adipose tissue to attenuate metabolic disorders by activating sirtuin 3
Acta Pharmacologica Sinica (2022)
-
Co-delivery of chemotherapeutic drugs and cell cycle regulatory agents using nanocarriers for cancer therapy
Science China Materials (2021)
-
Anticancer Activities and Mechanism of Action of Nagilactones, a Group of Terpenoid Lactones Isolated from Podocarpus Species
Natural Products and Bioprospecting (2020)