Abstract
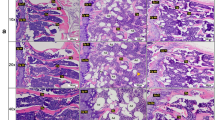
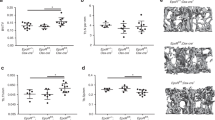
Recent studies demonstrate that diet quercetin (Quer) has obvious bone protective effects on ovariectomized rodents but thus far there is no direct evidence to support the inhibitory effect of Quer on bone loss caused by long-term unloading. In the present study, we investigated whether Quer could prevent bone loss induced by unloading in mice. Mice were subjected to hindlimb suspension (HLS) and received Quer (25, 50, 100 mg· kg−1 ·day−1, ig) for 4 weeks. Before euthanasia blood sample was collected; the femurs were harvested and subjected to MicroCT analysis. We showed that Quer administration markedly improved bone microstructure evidenced by dose-dependently reversing the reduction in bone volume per tissue volume, trabecular number, and bone mineral density, and the increase of trabecular spacing in mice with HLS. Analysis of serum markers and bone histometric parameters confirmed that Quer at both middle and high doses significantly decreased bone resorption-related markers collagen type I and tartrate-resistant acid phosphatase 5b, and increased bone formation-related marker procollagen 1 N-terminal propeptide as compared with HLS group. Treatment with Quer (1, 2, 5 μM) dose-dependently inhibited RANKL-induced osteoclastogenesis through promoting the expression of antioxidant hormone stanniocalcin 1 (STC1) and decreasing ROS generation; knockdown of STC1 blocked the inhibitory effect of Quer on ROS generation. Knockdown of STC1 also significantly promoted osteoclastogenesis in primary osteoclasts. In conclusion, Quer protects bones and prevents unloading-caused bone loss in mice through STC1-mediated inhibition of osteoclastogenesis. The findings suggest that Quer has the potential to prevent and treat off-load bone loss as an alternative supplement.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Lam H, Qin Y. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–100.
DeLong A, Friedman MA, Tucker SM, Krause AR, Kunselman A, Donahue HJ, et al. Protective effects of controlled mechanical loading of bone in C57BL6/J mice subject to disuse. JBMR. 2019;4:e10322.
Lloyd SA, Lang CH, Zhang Y, Paul EM, Laufenberg LJ, Lewis GS, et al. Interdependence of muscle atrophy and bone loss induced by mechanical unloading. J Bone Miner Res. 2014;29:1118–30.
Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, et al. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–29.
Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15:767–78.
Southmayd EA, Hellmers AC, De Souza MJ. Food versus pharmacy: assessment of nutritional and pharmacological strategies to improve bone health in energy-deficient exercising women. Curr Osteoporos Rep. 2017;15:459–72.
Leblanc A, Matsumoto T, Jones J, Shapiro J, Lang T, Shackelford L, et al. Bisphosphates as a supplement to exercise to protect bone during long-duration spaceflight. Osteoporos Int. 2013;24:2105–14.
Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50.
Chen JS, Sambrook PN. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 2011;8:81–91.
Haas AV, LeBoff MS. Osteoanabolic agents for osteoporosis. J Endocr Soc. 2018;2:922–32.
An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, et al. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016;147:46–58.
Wang T, Liu Q, Tjhioe W, Zhao J, Lu A, Zhang G, et al. Therapeutic potential and outlook of alternative medicine for osteoporosis. Curr Drug Targets. 2017;18:1051–68.
Shen F, Zhong H, Ge W, Ren J, Wang X. Quercetin/chitosan-graft-alpha lipoic acid micelles: a versatile antioxidant water dispersion with high stability. Carbohydr Polym. 2020;234:115927.
Bischoff SC. Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care. 2008;11:733–40.
Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, et al. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8:E529.
Ou QW, Zheng ZF, Zhao YY, Lin WQ. Impact of quercetin on systemic levels of inflammation: a meta-analysis of randomised controlled human trials. Int J Food Sci Nutr. 2020;71:152–63.
Ahmad N, Banala VT, Kushwaha P, Karvande A, SharmaS, Tripathi AK, et al. Quercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: a preventive strategy for post-menopausal osteoporosis. RSC Adv. 2016;6:97613–28.
Tsuji M, Yamamoto H, Sato T, Mizuha Y, Kawai Y, Taketani Y, et al. Dietary quercetin inhibits bone loss without effect on the uterus in ovariectomized mice. J Bone Miner Metab. 2009;27:673–81.
Zhou YN, Wu YQ, Ma WD, Jiang XQ, Takemra A, Uemura M, et al. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J Mater Chem B. 2017;5:612–25.
Yuan Z, Min J, Zhao YW, Cheng QF, Wang K, Lin SJ, et al. Quercetin rescued TNF-alpha-induced impairments in bone marrow-derived mesenchymal stem cell osteogenesis and improved osteoporosis in rats. Am J Transl Res. 2018;10:4313–21.
Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–77.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86.
Brodt MD, Ellis CB, Silva MJ. Growing C57Bl/6 mice increase whole bone mechanical properties by increasing geometric and material properties. J Bone Miner Res. 1999;14:2159–66.
Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608.
Tang SY, Herber RP, Ho SP, Alliston T. Matrix metalloproteinase-13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. J Bone Miner Res. 2012;27:1936–50.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8.
Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, et al. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J Cell Biochem. 2004;92:285–95.
Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896–906.
Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4740–7.
Agati G, Azzarello E, Pollastri S, Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76.
Pandhair V, Sekhon BS. Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biotechnol. 2006;15:71–8.
Qureshi MK, Munir S, Shahzad AN, Rasul S, Nouman W, Aslam K. Role of reactive oxygen species and contribution of new players in defense mechanism under drought stress in rice. Int J Agric Biol. 2018;20:1339–52.
Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Ucer SS, et al. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun. 2014;5:3773.
AlQranei MS, Aljohani H, Majumdar S, Senbanjo LT, Chellaiah MA. C-phycocyanin attenuates RANKL-induced osteoclastogenesis and bone resorption in vitro through inhibiting ROS levels, NFATc1 and NF-κB activation. Sci Rep. 2020;10:2513.
Yoshiko Y, Aubin JE, Maeda N. Stanniocalcin 1 (STC1) protein and mRNA are developmentally regulated during embryonic mouse osteogenesis: the potential of stc1 as an autocrine/paracrine factor for osteoblast development and bone formation. J Histochem Cytochem. 2002;50:483–92.
Terra SR, Cardoso JC, Félix RC, Martins LA, Souza DO, Guma FC, et al. STC1 interference on calcitonin family of receptors signaling during osteoblastogenesis via adenylate cyclase inhibition. Mol Cell Endocrinol. 2015;403:78–87.
Brum AM, van de Peppel J, Nguyen L, Aliev A, Schreuders-Koedam M, Gajadien T, et al. Using the connectivity map to discover compounds influencing human osteoblast differentiation. J Cell Physiol. 2018;233:4895–906.
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901.
Zenger S, Hollberg K, Ljusberg J, Norgård M, Ek-Rylander B, Kiviranta R, et al. Proteolytic processing and polarized secretion of tartrate-resistant acid phosphatase is altered in a subpopulation of metaphyseal osteoclasts in cathepsin K-deficient mice. Bone. 2007;41:820–32.
Acknowledgements
This study was financially supported by grants from the Natural Science Foundation of Shaanxi Province (Nos. 2020JM-128 and 2019JM-260), the Fundamental Research Funds for the Central Universities (No. 3102017zy051), and the Graduate Creative Innovation Seed Fund of Northwestern Polytechnical University (No. ZZ2019271).
Author information
Authors and Affiliations
Contributions
YBN, YHL, and QBM designed the experiments. YBN wrote the paper. YHL, and YMZ, revised the paper. YYY, XX, YS, YHZ, and DD, performed the experiments. YYY, XX, CRL, and XLW, analyzed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Niu, Yb., Yang, Yy., Xiao, X. et al. Quercetin prevents bone loss in hindlimb suspension mice via stanniocalcin 1-mediated inhibition of osteoclastogenesis. Acta Pharmacol Sin 41, 1476–1486 (2020). https://doi.org/10.1038/s41401-020-00509-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00509-z
Keywords
This article is cited by
-
miR-208a-3p discriminates osteoporosis, predicts fracture, and regulates osteoclast activation through targeting STC1
Journal of Orthopaedic Surgery and Research (2025)
-
Quercetins efficacy on bone and inflammatory markers, body composition, and physical function in postmenopausal women
Journal of Bone and Mineral Metabolism (2025)
-
Investigation of the effects of eugenol and quercetin on bone loss in STZ-NA induced diabetic rats utilizing micro CT
Journal of Diabetes & Metabolic Disorders (2022)