Abstract
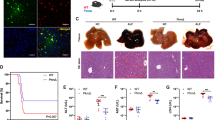
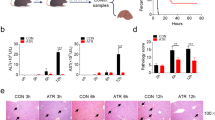
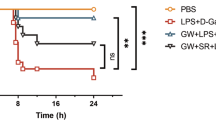
Acute liver failure (ALF) is a fatal clinical syndrome with no special drug. Recent evidence shows that modulation of macrophage to inhibit inflammation may be a promising strategy for ALF treatment. In this study we investigated the potential therapeutic effects of melittin, a major peptide component of bee venom both in mice model of ALF and in LPS-stimulated macrophages in vitro, and elucidated the underlying mechanisms. ALF was induced in mice by intraperitoneal injection of d-galactosamine/LPS. Then the mice were treated with melittin (2, 4, and 8 mg/kg, ip). We showed that melittin treatment markedly improved mortality, attenuated severe symptoms and signs, and alleviated hepatic inflammation in d-galactosamine/LPS-induced ALF mice with the optimal dose being 4 mg/kg. In addition, melittin within the effective doses did not cause significant in vivo toxicity. In LPS-stimulated RAW264.7 macrophages, melittin (0.7 μM) exerted anti-oxidation and anti-inflammation effects. We showed that LPS stimulation promoted aerobic glycolysis of macrophages through increasing glycolytic rate, upregulated the levels of Warburg effect-related enzymes and metabolites including lactate, LDHA, LDH, and GLUT-1, and activated Akt/mTOR/PKM2/HIF-1α signaling. Melittin treatment suppressed M2 isoform of pyruvate kinase (PKM2), thus disrupted the Warburg effect to alleviate inflammation. Molecular docking analysis confirmed that melittin targeted PKM2. In LPS-stimulated RAW264.7 macrophages, knockdown of PKM2 caused similar anti-inflammation effects as melittin did. In d-galactosamine/LPS-induced ALF mice, melittin treatment markedly decreased the expression levels of PKM2 and HIF-1α in liver. This work demonstrates that melittin inhibits macrophage activation-mediated inflammation via inhibition of aerobic glycolysis by targeting PKM2, which highlights a novel strategy of using melittin for ALF treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Flamm SL, Yang YX, Singh S, Falck-Ytter YT, AGA Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guidelines for the diagnosis and management of acute liver failure. Gastroenterology. 2017;152:644–7.
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525–34.
Ferriero R, Nusco E, De Cegli R, Carissimo A, Manco G, Brunetti-Pierri N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute. J Hepatol. 2018;S0168-8278:30210–1.
O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663–70.
Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734–9.
Xia XM, Fu JL, Song XF, Shi Q, Su CY, Song EQ, et al. Neohesperidin dihydrochalcone down-regulates MyD88-dependent and -independent signaling by inhibiting endotoxin-induced trafficking of TLR4 to lipid rafts. Free Radic Biol Med. 2015;89:522–32.
Puengel T, Tacke F. Repair macrophages in acute liver failure. Gut. 2018;67:202–3.
Zhou RR, Zhao SS, Zou MX, Zhang P, Zhang BX, Dai XH, et al. HMGB1 cytoplasmic translocation in patients with acute liver failure. BMC Gastroenterol. 2011;11:21.
Ehud Z, Shany SG, Metsada PC, Eli B, Oren S, Zamir H, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–53.
You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and in filtrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86:836–43.
Bernal W, Lee WM, Wendon J, Larsen FS, Williams R. Acute liver failure: a curable disease by 2024? J Hepatol. 2015;62:S112–20.
Possamai LA, Thursz MR, Wendon JA, Antoniades CG. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61:439–45.
Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–12.
Tak E, Jung DH, Kim SH, Park GC, Jun DY, Lee J, et al. Protective role of hypoxia-inducible factor-1α-dependent CD39 and CD73 in fulminant acute liver failure. Toxicol Appl Pharmacol. 2017;314:72–81.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science. 2017;356:513–9.
Meiser J, Krämer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, et al. Pro-inflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J Biol Chem. 2016;291:3932–46.
Yang LC, Xie M, Yang MH, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014;5:4436.
Zhang ZX, Deng WJ, Kang R, Xie M, Billiar T, Wang HC, et al. Plumbagin protects mice from lethal sepsis by modulating immunometabolism upstream of PKM2. Mol Med. 2016;22:162–72.
Corcoran SE, O’Neill LA. HIF-1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126:3699–707.
Luo WB, Hu HX, Chang R, Zhong J, Knabel M, O’Meally R, et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44.
Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35:965–73.
Liu N, Parry S, Xiao Y, Zhou S, Liu Q. Molecular targets of the Warburg effect and inflammatory cytokines in the pathogenesis of pulmonary artery hypertension. Clin Chim Acta. 2017;466:98–104.
Rady I, Siddiqui IA, Rady M, Mukhtar H. Melittin, a major peptide component of bee venom, and its conjugates in cancer therapy. Cancer Lett. 2017;402:16–31.
An HJ, Kim JY, Kim WH, Gwon MG, Gu HM, Jeon MJ, et al. Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br J Pharmacol. 2018;175:4310–24.
Al-Ani I, Zimmermann S, Reichling J, Wink M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine. 2015;22:245–55.
Park JH, Kim KH, Lee WR, Han SM, Park KK. Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis. 2012;17:61–9.
Farghali H, Kgalalelo Kemelo M, Wojnarová L, Kutinová Canová N. In vitro and in vivo experimental hepatotoxic models in liver research: applications to the assessment of potential hepatoprotective drugs. Physiol Res. 2016;65:S417–25.
Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, et al. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001;33:1441–50.
Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–52.
Reddy DB, Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;381:112–7.
Wang HJ, Hsieh YJ, Cheng WC, Lin CP, Lin YS, Yang SF, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc Natl Acad Sci U S A. 2014;111:279–84.
Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108:4129–34.
Kim WH, An HJ, Kim JY, Gwon MG, Gu H, Jeon M, et al. Beneficial effects of melittin on ovalbumin-induced atopic dermatitis in mouse. Sci Rep. 2017;7:17679.
Park SY, Park DJ, Kim YH, Kim YH, Choi YW, Lee SJ, et al. Schisandra chinensis alpha-iso-cubebenol induces heme oxygenase-1 expression through PI3K/Akt and Nrf2 signaling and has anti-inflammatory activity in Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2011;11:1907–15.
Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, et al. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007;7:1092–101.
Jia HR, Zhu YX, Xu KF, Wu FG. Turning toxicants into safe therapeutic drugs: cytolytic peptide-photosensitizer assemblies for optimized in vivo delivery of melittin. Adv Healthc Mater. 2018;7:e1800380.
Xie M, Yu Y, Kang R, Zhu S, Yang LC, Zeng L, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280.
Acknowledgements
This work was supported by the project funded by China Postdoctoral Science Foundation (no’s. 2016M600639 and 2017T100614), the National Natural Science Foundation of China (no’s. 81673719, 81970550, 81700561 and 81873574), and Natural Science Foundation of Hunan Province (no’s. 2019JJ30042 and 11JJ6072).
Author information
Authors and Affiliations
Contributions
NL performed animal experiments and wrote the manuscript; DZ, SYP, and PCZ performed the cell experiments; XWH and TL performed the molecular docking analysis; ZBH and YH wrote part of the manuscript; XGF designed the experiments and edited the manuscript; YW and XGF designed the experiments, analyzed the data, XGF and SYP revised all figures, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Fan, Xg., Pei, Sy., Zhou, D. et al. Melittin ameliorates inflammation in mouse acute liver failure via inhibition of PKM2-mediated Warburg effect. Acta Pharmacol Sin 42, 1256–1266 (2021). https://doi.org/10.1038/s41401-020-00516-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00516-0
Keywords
This article is cited by
-
PKM2-mediated metabolic reprogramming of microglia in neuroinflammation
Cell Death Discovery (2025)
-
SULT2B1: a novel therapeutic target in colorectal cancer via modulation of AKT/PKM2-mediated glycolysis and proliferation
Journal of Translational Medicine (2024)
-
PKM2-mediated STAT3 phosphorylation promotes acute liver failure via regulating NLRP3-dependent pyroptosis
Communications Biology (2024)
-
Neuroprotective Effects of Melittin Against Cerebral Ischemia and Inflammatory Injury via Upregulation of MCPIP1 to Suppress NF-κB Activation In Vivo and In Vitro
Neurochemical Research (2024)
-
CB2R agonist GW405833 alleviates acute liver failure in mice via inhibiting HIF-1α-mediated reprogramming of glycometabolism and macrophage proliferation
Acta Pharmacologica Sinica (2023)