Abstract
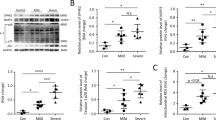
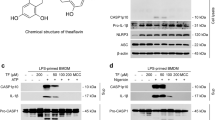
Metabolic reprogramming is associated with NLRP3 inflammasome activation in activated macrophages, contributing to inflammatory responses. Tanshinone IIA (Tan-IIA) is a major constituent from Salvia miltiorrhiza Bunge, which exhibits anti-inflammatory activity. In this study, we investigated the effects of Tan-IIA on inflammation in macrophages in focus on its regulation of metabolism and redox state. In lipopolysaccharides (LPS)-stimulated mouse bone marrow-derived macrophages (BMDMs), Tan-IIA (10 μM) significantly decreased succinate-boosted IL-1β and IL-6 production, accompanied by upregulation of IL-1RA and IL-10 release via inhibiting succinate dehydrogenase (SDH). Tan-IIA concentration dependently inhibited SDH activity with an estimated IC50 of 4.47 μM in LPS-activated BMDMs. Tan-IIA decreased succinate accumulation, suppressed mitochondrial reactive oxygen species production, thus preventing hypoxia-inducible factor-1α (HIF-1α) induction. Consequently, Tan-IIA reduced glycolysis and protected the activity of Sirtuin2 (Sirt2), an NAD+-dependent protein deacetylase, by raising the ratio of NAD+/NADH in activated macrophages. The acetylation of α-tubulin was required for the assembly of NLRP3 inflammasome; Tan-IIA increased the binding of Sirt2 to α-tubulin, and thus reduced the acetylation of α-tubulin, thus impairing this process. Sirt2 knockdown or application of Sirt2 inhibitor AGK-2 (10 μM) neutralized the effects of Tan-IIA, suggesting that Tan-IIA inactivated NLRP3 inflammasome in a manner dependent on Sirt2 regulation. The anti-inflammatory effects of Tan-IIA were observed in mice subjected to LPS challenge: pre-administration of Tan-IIA (20 mg/kg, ip) significantly attenuated LPS-induced acute inflammatory responses, characterized by elevated IL-1β but reduced IL-10 levels in serum. The peritoneal macrophages isolated from the mice displayed similar metabolic regulation. In conclusion, Tan-IIA reduces HIF-1α induction via SDH inactivation, and preserves Sirt2 activity via downregulation of glycolysis, contributing to suppression of NLRP3 inflammasome activation. This study provides a new insight into the anti-inflammatory action of Tan-IIA from the respect of metabolic and redox regulation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Murray Peter J, Allen Judith E, Biswas Subhra K, Fisher Edward A, Gilroy Derek W, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20.
Kumar, V. Macrophages: The potent immunoregulatory innate immune cells. In: Bhat, KH (ed.). In macrophage activation-biology and disease. London, UK: IntechOpen, 2020.
El Kasmi KC, Stenmark KR. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol. 2015;27:267–75.
Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–84.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Langston PK, Shibata M, Horng T. Metabolism supports macrophage activation. Front Immunol. 2017;8:61. https://doi.org/10.3389/fimmu.2017.00061.
O’Neill Luke AJ. A broken Krebs cycle in macrophages. Immunity. 2015;42:393–4.
Mills E, O’Neill LAJ. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–20.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50.
Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–70.e13.
Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–42.
Corcoran SE, O’Neill LAJ. HIF1α and metabolic reprogramming in inflammation. J Clin Investig. 2016;126:3699–707.
Ryan DG, Murphy MP, Frezza C, Prag HA, Chouchani ET, O'Neill LA, et al. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat Metab. 2019;1:16–33.
Brüne B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, et al. Redox control of inflammation in macrophages. Antioxid Redox Sign. 2013;19:595–637.
Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–13.
Chalkiadaki A, Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 2012;8:287–96.
Verdin E, Hirschey MD, Finley LWS, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–75.
Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med. 2012;52:281–90.
Gomes P, Fleming Outeiro T, Cavadas C. Emerging role of Sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol Sci. 2015;36:756–68.
Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–61.
Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013;14:454–60.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
Shang Q, Xu H, Huang L. Tanshinone IIA: a promising natural cardioprotective agent. Evid Based Complement Alternat Med. 2012;2012:716459. https://doi.org/10.1155/2012/716459.
Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10.
Xu W, Yang J, Wu LM. Cardioprotective effects of tanshinone IIA on myocardial ischemia injury in rats. Pharmazie. 2009;64:332–6.
Fan G, Jiang X, Wu X, Fordjour PA, Miao L, Zhang H, et al. Anti-inflammatory activity of tanshinone IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and TLR4-NF-kappaB pathway. Inflammation. 2016;39:375–84.
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu LM, et al. The anti-inflammatory activities of tanshinone IIA, an active component of TCM, are mediated by estrogen receptor activation and inhibition of iNOS. J Steroid Biochem. 2009;113:275–80.
Feng J, Li S, Chen H. Tanshinone IIA inhibits myocardial remodeling induced by pressure overload via suppressing oxidative stress and inflammation: possible role of silent information regulator 1. Eur J Pharmacol. 2016;791:632–9.
Shu M, Hu XR, Hung ZA, Huang DD, Zhang S. Effects of tanshinone IIA on fibrosis in a rat model of cirrhosis through heme oxygenase-1, inflammation, oxidative stress and apoptosis. Mol Med Rep. 2016;13:3036–42.
Baolin L, Inami Y, Tanaka H, Inagaki N, Iinuma M, Nagai H. Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Med. 2004;70:305–9.
Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6.
Zhao YP, Wang F, Jiang W, Liu J, Liu BL, Qi LW, et al. A mitochondrion-targeting tanshinone IIA derivative attenuates myocardial hypoxia reoxygenation injury through a SDH-dependent antioxidant mechanism. J Drug Target. 2019;27:896–902.
Muendlein HI, Poltorak A. Flipping the switch from inflammation to cell death. Trends Immunol. 2020;41:648–51.
Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. 2016;1857:1086–101.
Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol. 2016;46:13–21.
Sanin DE, Matsushita M, Klein Geltink RI, Grzes KM, van Teijlingen Bakker N, Corrado M, et al. Mitochondrial membrane potential regulates nuclear gene expression in macrophages exposed to prostaglandin E2. Immunity. 2018;49:1021–33.e6.
Hirst J, King M, Pryde K. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–80.
An L, Peng LY, Sun NY, Yang YL, Zhang XW, Li B, et al. Tanshinone IIA activates nuclear factor-erythroid 2-related factor 2 to restrain pulmonary fibrosis via regulation of redox homeostasis and glutaminolysis. Antioxid Redox Sign. 2019;30:1831–48.
Sharma M, Boytard L, Hadi T, Koelwyn G, Simon R, Ouimet M, et al. Enhanced glycolysis and HIF-1α activation in adipose tissue macrophages sustains local and systemic interleukin-1β production in obesity. Sci Rep. 2020;10:5555.
Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell. 2005;7:77–85.
Liu T, Yang W, Pang S, Yu S, Yan B. Functional genetic variants within the SIRT2 gene promoter in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2018;137:200–7.
Anwar T, Khosla S, Ramakrishna G. Increased expression of SIRT2 is a novel marker of cellular senescence and is dependent on wild type p53 status. Cell Cycle. 2016;15:1883–97.
Flick F, Lüscher B. Regulation of sirtuin function by posttranslational modifications. Front Pharmacol. 2012;3:29. https://doi.org/10.3389/fphar.2012.00029.
Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95.
Jiang D, Chen S, Sun R, Zhang X, Wang D. The NLRP3 inflammasome: role in metabolic disorders and regulation by metabolic pathways. Cancer Lett. 2018;419:8–19.
Zhang WL, Cao YA, Xia J, Tian L, Yang L, Peng CS. Neuroprotective effect of tanshinone IIA weakens spastic cerebral palsy through inflammation, p38MAPK and VEGF in neonatal rats. Mol Med Rep. 2018;17:2012–8.
Acknowledgements
We thank all the co-authors who participated in our present study. The present work was supported by the Fundamental Research Funds for the Central Universities (2632019ZD02), “Double First-Class” University project (CPU2018GF07), and Natural Science Foundation of Jiangsu Province (BK20191323).
Author information
Authors and Affiliations
Contributions
QYL and YZ participated in the most of experimental performance, data analysis, and the preparation of the paper. QYL, YZ, XRS, QN, and QSS performed the animal experiments, primary BMDMs isolation and culture, the qPCR, Western blot analysis, immunoprecipitation and immunohistochemical analysis, etc. XNL and BLL were mainly involved in the improvement of paper. NL provided the technical support in sample preparation and data analysis. BLL, FH, and ZXQ designed and supervised the study, and revised the paper. All the authors approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Qy., Zhuang, Y., Song, Xr. et al. Tanshinone IIA prevents LPS-induced inflammatory responses in mice via inactivation of succinate dehydrogenase in macrophages. Acta Pharmacol Sin 42, 987–997 (2021). https://doi.org/10.1038/s41401-020-00535-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00535-x
Keywords
This article is cited by
-
A Hypoxia-Inflammation Cycle and Multiple Sclerosis: Mechanisms and Therapeutic Implications
Current Treatment Options in Neurology (2025)
-
Local Application of Tanshinone IIA protects mesenchymal stem cells from apoptosis and promotes fracture healing in ovariectomized mice
Journal of Orthopaedic Surgery and Research (2024)
-
Herbacetin ameliorates lipopolysaccharide-elicited inflammatory response by suppressing NLRP-3/AIM-2 inflammasome activation, PI3K/Akt/MAPKs/NF-κB redox inflammatory signalling, modulating autophagy and macrophage polarization imbalance
Molecular Biology Reports (2024)
-
Metabolite itaconate in host immunoregulation and defense
Cellular & Molecular Biology Letters (2023)
-
Integrated microbiome-metabolome-genome axis data of Laiwu and Lulai pigs
Scientific Data (2023)