Abstract
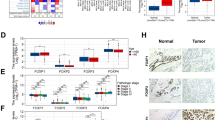
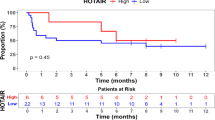
Triple-negative breast cancer (TNBC) is characterized by low expression of human epidermal growth factor receptor-2 (HER2), estrogen receptor (ER), and progesterone receptor (PR), which is the most aggressive subtype with poor outcome among breast cancers. The underlying mechanisms of TNBC remain unclear and there is a lack of biomarkers. In this study we conducted an in silico assay and found that FOXC1 was highly expressed in ER−/PR−/HER2− breast cancers, which was confirmed by qRT-PCR, immunohistochemistry, and Western blot analysis. FOXC1 was more highly expressed in TNBCs than the other breast cancers. Kaplan–Meier plotter revealed that expression of FOXC1 was associated with overall survival (OS) of patients with breast cancers. Expression of FOXC1 was reversely associated with level of H3K27me3, which was methylated by EZH2. In MCF-7 and T47D cells, inhibition of EZH2 by DZNeP or GSK343 concentration- and time-dependently increased expression of FOXC1. Finally, we demonstrated that the expression of FOXC1 was associated with resistance of doxorubicin treatment of breast cancer cells. In conclusion, these results suggest that FOXC1 may be a potential biomarker or drug target for TNBCs, and that downregulation of FOXC1 could have therapeutic value in treatment of TNBCs.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Wang J, Li W, Zhao Y, Kang FuW, Zheng X, et al. Members of FOX family could be drug targets of cancers. Pharmacol Ther. 2018;181:183–96.
Wang J, Li W, Zheng X, Pang X, Du G. Research progress on the forkhead box C1. Oncotarget. 2018;9:12471–8.
Nishimura DY, Swiderski RE, Alward WL, Searby CC, Patil SR, Bennet SR, et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 1998;19:140–7.
Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 1998;63:1316–28.
Komatireddy S, Chakrabarti S, Mandal AK, Reddy AB, Sampath S, Panicker SG, et al. Mutation spectrum of FOXC1 and clinical genetic heterogeneity of Axenfeld-Rieger anomaly in India. Mol Vis. 2003;9:43–8.
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–6.
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, et al. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-κB signaling. Oncogene. 2012;31:4798–802.
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z. et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–24.
Wei LX, Zhou RS, Xu HF, Wang JY, Yuan MH. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumour Biol J Int Soc Oncodev Biol Med. 2013;34:941–6.
Xu Y, Shao QS, Yao HB, Jin Y, Ma YY, Jia LH. Overexpression of FOXC1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2014;64:963–70.
Somerville TD, Wiseman DH, Spencer GJ, Huang X, Lynch JT, Leong HS, et al. Frequent derepression of the mesenchymal transcription factor gene FOXC1 in acute myeloid leukemia. Cancer Cell. 2015;28:329–42.
Wang J, Li L, Liu S, Zhao Y, Wang L, Du G. FOXC1 promotes melanoma by activating MST1R/PI3K/AKT. Oncotarget. 2016;7:84375–87.
Wang L, Gu F, Liu CY, Wang RJ, Li J, Xu JY. High level of FOXC1 expression is associated with poor prognosis in pancreatic ductal adenocarcinoma. Tumour Biol J Int Soc Oncodev Biol Med. 2013;34:853–8.
Pan F, Yao J, Chen Y, Zhou C, Geng P, Mao H, et al. A novel long non-coding RNA FOXCUT and mRNA FOXC1 pair promote progression and predict poor prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:2838–49.
van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103:1574–80.
Sonne SB, Yadav R, Yin G, Dalgaard MD, Myrmel LS, Gupta R, et al. Obesity is associated with depot-specific alterations in adipocyte DNA methylation and gene expression. Adipocyte. 2017;6:124–33.
Vasudevan D, Hickok JR, Bovee RC, Pham V, Mantell LL, Bahroos N, et al. Nitric oxide regulates gene expression in cancers by controlling histone posttranslational modifications. Cancer Res. 2015;75:5299–308.
Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11.
Gall Trošelj K, Novak Kujundzic R, Ugarkovic D. Polycomb repressive complex’s evolutionary conserved function: the role of EZH2 status and cellular background. Clin Epigenetics. 2016;8:55.
Yoo KH, Hennighausen L. EZH2 methyltransferase and H3K27 methylation in breast cancer. Int J Biol Sci. 2012;8:59–65.
Wang J, Kuiatse I, Lee AV, Pan J, Giuliano A, Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelial-mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal-regulated kinase activation. Mol Cancer Res MCR. 2010;8:266–77.
Qu Y, Wang J, Ray PS, Guo H, Huang J, Shin-Sim M, et al. Thioredoxin-like 2 regulates human cancer cell growth and metastasis via redox homeostasis and NF-κB signaling. J Clin Invest. 2011;121:212–25.
Wang J, Huang SK, Marzese DM, Hsu SC, Kawas NP, Chong KK, et al. Epigenetic changes of EGFR have an important role in BRAF inhibitor-resistant cutaneous melanomas. J Invest Dermatol. 2015;135:532–41.
Kaemmerer D, Peter L, Lupp A, Schulz S, Sanger J, Baum RP, et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5:187–94.
Specht E, Kaemmerer D, Sanger J, Wirtz RM, Schulz S, Lupp A. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67:368–77.
Yang Y, Guan D, Lei L, Lu J, Liu JQ, Yang G, et al. H6, a novel hederagenin derivative, reverses multidrug resistance in vitro and in vivo. Toxicol Appl Pharmacol. 2018;341:98–105.
Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, et al. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17:197–209.
Khosravi-Shahi P, Cabezón-Gutiérrez L, Aparicio Salcedo MI. State of art of advanced triple negative breast cancer. Breast J. 2019;25:967–70.
Praestegaard C, Kjaer SK, Andersson M, Steding-Jensen M, Frederiksen K, Mellemkjaer L. Risk of skin cancer following tamoxifen treatment in more than 16,000 breast cancer patients: a cohort study. Breast Cancer. 2016;23:908–16.
Han J, Lim W, You D, Jeong Y, Kim S, Lee JE, et al. Chemoresistance in the human triple-negative breast cancer cell line MDA-MB-231 induced by doxorubicin gradient is associated with epigenetic alterations in histone deacetylase. J Oncol. 2019;2019:1345026.
Wein L, Loi S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast. 2017;34 Suppl 1:S27–30.
Wang J, Xu Y, Li L, Wang L, Yao R, Sun Q, et al. FOXC1 is associated with estrogen receptor alpha and affects sensitivity of tamoxifen treatment in breast cancer. Cancer Med. 2017;6:275–87.
Tang L, Jin J, Xu K, Wang X, Tang J, Guan X. SOX9 interacts with FOXC1 to activate MYC and regulate CDK7 inhibitor sensitivity in triple-negative breast cancer. Oncogenesis. 2020;9:47.
Huang Y, Huang H, Li M, Zhang X, Liu Y, Wang Y. MicroRNA-374c-5p regulates the invasion and migration of cervical cancer by acting on the Foxc1/snail pathway. Biomed Pharmacother. 2017;94:1038–47.
Han B, Qu Y, Jin Y, Yu Y, Deng N, Wawrowsky K, et al. FOXC1 activates smoothened-independent hedgehog signaling in basal-like breast cancer. Cell Rep. 2015;13:1046–58.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81573454 to JHW, No. 81703536 to WL) and the Beijing Natural Science Foundation of Beijing Municipality (7172142). This work was also supported by the CAMS Innovation Fund for Medical Sciences (2016-I2M-3-007) and the Technology Major Projects for “Major New Drugs Innovation and Development” (2018ZX09711001-005-025, 2018ZX09711001-012).
Author information
Authors and Affiliations
Contributions
JHW and GHD developed the hypothesis, designed the experiments, and revised the manuscript. XJZ and WL conducted all experiments and wrote the main manuscript. JYL, LWR, SWL, and XMZ collected data and performed the statistical analysis. JY provided the study materials and patients. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, Xj., Li, W., Yi, J. et al. EZH2 regulates expression of FOXC1 by mediating H3K27me3 in breast cancers. Acta Pharmacol Sin 42, 1171–1179 (2021). https://doi.org/10.1038/s41401-020-00543-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00543-x
Keywords
This article is cited by
-
EZH1/2 as targets for cancer therapy
Cancer Gene Therapy (2023)
-
The role of FOXC1/FOXCUT/DANCR axis in triple negative breast cancer: a bioinformatics and experimental approach
Molecular Biology Reports (2022)
-
DZNep promotes mouse bone defect healing via enhancing both osteogenesis and osteoclastogenesis
Stem Cell Research & Therapy (2021)