Abstract
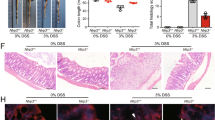
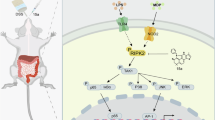
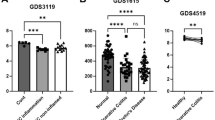
Endoplasmic reticulum (ER) homeostasis is regulated by ER-resident E3 ubiquitin ligase Hrd1, which has been implicated in inflammatory bowel disease (IBD). Ginsenoside Rb1 (GRb1) is the major ginsenoside in ginseng with multiple pharmacological activities. In this study we investigated the role of Hrd1 in IBD and its regulation by GRb1. Two mouse colitis models were established to mimic human IBD: drinking water containing dextran sodium sulfate (DSS) as well as intra-colonic infusion of 2, 4, 6-trinitrobenzene sulfonic acid (TNBS). Colitis mice were treated with GRb1 (20, 40 mg·kg−1·d−1, ig) or a positive control drug sulfasalazine (500 mg·kg−1·d−1, ig) for 7 days. The model mice showed typical colitis symptoms and pathological changes in colon tissue. In addition to significant inflammatory responses and cell apoptosis in colon tissue, colon epithelial expression of Hrd1 was significantly decreased, the expression of ER stress markers GRP78, PERK, CHOP, and caspase 12 was increased, and the expression of Fas was increased (Fas was removed by Hrd1-induced ubiquitination). These changes were partially, or completely, reversed by GRb1 administration, whereas injection of Hrd1 inhibitor LS102 (50 mg·kg−1· d−1, ip, for 6 days) exacerbated colitis symptoms in colitis mice. GRb1 administration not only normalized Hrd1 expression at both the mRNA and protein levels, but also alleviated the ER stress response, Fas-related apoptosis, and other colitis symptoms. In intestinal cell line IEC-6, the expression of Hrd1 was significantly decreased by LPS treatment, but was normalized by GRb1 (200 μM). GRb1 alleviated LPS-induced ER stress and cell apoptosis in IEC-6 cells, and GRb1 action was inhibited by knockdown of Hrd1 using small interfering RNA. In summary, these results reveal a pathological role of Hrd1 in colitis, and provide a novel insight into alternative treatment of colitis using GRb1 activating Hrd1 signaling pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol. 2013;35:229–44.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2011;142:46–54.e42.
Na Z, Xiuqin Z, Xifei H, Ru C, Yanqiu M. Bibliometric analysis of quality of life in 473 patients with inflammatory bowel disease from 2006 to 2016 in China. Chin J Mod Nurs. 2018;24:309–13.
Chiodini RJ, Dowd SE, Galandiuk S, Davis B, Glassing A. The predominant site of bacterial translocation across the intestinal mucosal barrier occurs at the advancing disease margin in Crohn’s disease. Microbiology. 2016;162:1608.
Theresa A, Osborne LC, Saenz SA, Dmytro K, Ziegler CGK, Mullican SE, et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153.
Lin JC, Wu JQ, Wang F, Tang FY, Sun J, Xu B, et al. QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 2019;52:e12547.
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–74.
Ruiz PA, Morón B, Becker HM, Lang S, Atrott K, Spalinger MR, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66:1216–24.
Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5:V1–16.
Hibi T, Fujiyama Y. Biological therapies for inflammatory bowel disease. J Gastroenterol. 2002;37:43.
Sugimoto S, Naganuma M, Kiyohara H, Arai M, Ono K, Mori K, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93:193.
Xu Y, Zhao F, Qiu Q, Chen K, Wei J, Kong Q, et al. The ER membrane-anchored ubiquitin ligase Hrd1 is a positive regulator of T-cell immunity. Nat Commun. 2016;7:12073.
Kong S, Yang Y, Xu Y, Wang Y, Zhang Y, Melo-Cardenas J, et al. Endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 controls B-cell immunity through degradation of the death receptor CD95/Fas. Proc Natl Acad Sci U S A. 2016;113:10394–9.
Yang H, Qiu Q, Gao B, Kong S, Lin Z, Fang D. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med. 2014;211:2467–79.
Hoelen H, Zaldumbide A, van Leeuwen WF, Torfs EC, Engelse MA, Hassan C, et al. Proteasomal degradation of proinsulin requires Derlin-2, HRD1 and p97. PLoS One. 2015;10:e0128206.
Amano T, Yamasaki S, Yagishita N, Tsuchimochi K, Shin H, Kawahara K, et al. Synoviolin/Hrd1, an E3 ubiquitin ligase, as a novel pathogenic factor for arthropathy. Genes Dev. 2003;17:2436–49.
Sun S, Lourie R, Cohen SB, Ji Y, Goodrich JK, Poole AC, et al. Epithelial Sel1L is required for the maintenance of intestinal homeostasis. Mol Biol Cell. 2015;27:483–90.
Kaser A, Lee A, Franke A, Glickman J, Zeissig S, Tilg H, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56.
Tréton X, Pédruzzi E, Cazals–Hatem D, Grodet A, Panis Y, Groyer A, et al. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology. 2011;141:1024–35.
Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, et al. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel disease pathogenesis: an update review. Front Immunol. 2017;8:1271.
Okuda-Shimizu Y, Hendershot LM. Characterization of an ERAD pathway for nonglycosylated BiP substrates, which require herp. Mol Cell. 2007;28:544–54.
Doroudgar S, Völkers M, Thuerauf DJ, Khan M, Mohsin S, Respress JL, et al. Hrd1 and ER-associated protein degradation, ERAD, are critical elements of the adaptive ER stress response in cardiac myocytes. Circ Res. 2015;117:536–46.
Shruthi K, Reddy SS, Reddy GB. Ubiquitin-proteasome system and ER stress in the retina of diabetic rats. Arch Biochem Biophys. 2017;627:10–20.
Tan SX, Jiang DX, Hu RC, Dai AG, Gan GX, Fu DY, et al. Endoplasmic reticulum stress induces HRD1 to protect alveolar type II epithelial cells from apoptosis induced by cigarette smoke extract. Cell Physiol Biochem. 2017;43:1337–45.
Tan S, Yu W, Lin Z, Chen Q, Shi J, Dong Y, et al. Anti-inflammatory effect of ginsenoside Rb1 contributes to the recovery of gastrointestinal motility in the rat model of postoperative ileus. Biol Pharm Bull. 2014;37:1788–94.
Oh SJ, Kim K, Lim CJ. Protective properties of ginsenoside Rb1 against UV-B radiation-induced oxidative stress in human dermal keratinocytes. Die Pharmazie. 2015;70:381–7.
Zhang YL, Liu Y, Kang XP, Dou CY, Zhuo RG, Huang SQ, et al. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson’s disease. Neuropharmacology. 2017;131:223–37.
Zhang J, Cao L, Wang H, Cheng X, Wang L, Zhu L, et al. Ginsenosides regulate PXR/NF-kappaB signaling and attenuate dextran sulfate sodium-induced colitis. Drug Metab Dispos. 2015;43:1181–9.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharm. 2010;160:1577–9.
Xiong Y, Shi L, Wang L, Zhou Z, Wang C, Lin Y, et al. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharm Res. 2017;123:73–82.
Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sci. 2013;92:708–18.
Rottgen TS, Nickerson AJ, Minor EA, Stewart AB, Harold AD, Rajendran VM. Dextran sulfate sodium-induced chronic colitis attenuates Ca2+-activated Cl− secretion in murine colon by downregulating TMEM16A. Am J Physiol Cell Physiol. 2018;315:C10–20.
Zhou P, Lu S, Luo Y, Wang S, Yang K, Zhai Y, et al. Attenuation of TNF-alpha-induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-kappaB, JNK and p38 signaling pathways. Front Pharmacol. 2017;8:464.
Bo Q, Lan Z, Zhiqun Z, Jiangqiong O, Hui H. Effects of ginsenosides-Rb1 on exercise-induced oxidative stress in forced swimming mice. Pharmacogn Mag. 2014;10:458–63.
Das UN. Inflammatory bowel disease as a disorder of an imbalance between pro- and anti-inflammatory molecules and deficiency of resolution bioactive lipids. Lipids Health Dis. 2016;15:11.
Lixuan S, Nan G, Bing C, Weixin L, Min J. Comparison of DSS, TNBS and OXZ-induced colitis in animal models. Chin J Gastroen Hepatol. 2013;22:1221–4.
Yang T, Miao Y, Zhang T, Mu N, Ruan L, Duan J, et al. Ginsenoside Rb1 inhibits autophagy through regulation of Rho/ROCK and PI3K/mTOR pathways in a pressure-overload heart failure rat model. J Pharm Pharmacol. 2018;70:830–8.
Wang J, Qiao L, Li Y, Yang G. Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion-induced liver injury by inhibiting NF-kappaB activation. Exp Mol Med. 2008;40:686–98.
Xu Y, Melo-Cardenas J, Zhang Y, Gau I, Wei J, Montauti E, et al. The E3 ligase Hrd1 stabilizes Tregs by antagonizing inflammatory cytokine-induced ER stress response. JCI Insight. 2019;4:e121887.
Dibdiakova K, Saksonova S, Pilchova I, Klacanova K, Tatarkova Z, Racay P. Both thapsigargin- and tunicamycin-induced endoplasmic reticulum stress increases expression of Hrd1 in IRE1-dependent fashion. Neurol Res. 2019;41:177–88.
Cao SS. Epithelial ER stress in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:984–93.
Adolph TE, Niederreiter L, Blumberg RS, Kaser A. IBD is a disorder of defective autophagy and innate immunity: endoplasmic reticulum stress and inflammation. Dig Dis. 2012;30:341.
Liu L, Long H, Wu Y, Li H, Dong L, Zhong JL, et al. HRD1-mediated PTEN degradation promotes cell proliferation and hepatocellular carcinoma progression. Cell Signal. 2018;50:90–9.
Huang Y, Sun Y, Cao Y, Sun H, Li M, You H, et al. HRD1 prevents apoptosis in renal tubular epithelial cells by mediating eIF2α ubiquitylation and degradation. Cell Death Dis. 2017;8:3202.
Wang Y, Guo A, Liang X, Li M, Shi M, Li Y, et al. HRD1 sensitizes breast cancer cells to Tamoxifen by promoting S100A8 degradation. Oncotarget. 2017;8:23564–74.
Cheng LQ, Kim MK, Lee JW, Lee YJ, Yang DC. Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett. 2006;28:1121–7.
Kim DY, Park MW, Yuan HD, Lee HJ, Kim SH, Chung SH. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57:10573–8.
Lee ES, Choi JS, Kim MS, You HJ, Ji GE, Kang YH. Ginsenoside metabolite compound K differentially antagonizing tumor necrosis factor-alpha-induced monocyte-endothelial trafficking. Chem Biol Interact. 2011;194:13–22.
Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang B, et al. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-kappaB activation. PLoS One. 2014;9:e87810.
Xu X, Lu Q, Wu J, Li Y, Sun J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Inflammopharmacology. 2017;25:33–40.
Chang WH, Tsai YL, Huang CY, Hsieh CC, Chaunchaiyakul R, Fang Y, et al. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J Int Soc Sports Nutr. 2015;12:34.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 81600440); basic research projects of Liaoning Province Universities (Grant No. LQ2017042); Dalian High Level Talent Support program (No. 2017RQ017); and National Key Research and Development Project of China (2017YFD050160203).
Author information
Authors and Affiliations
Contributions
DPC and JYW designed the research; JYD, KJX, WL, LLL, FY, XSF, YJX, LW, ZJZ, CYL, and WDZ performed the experiments; JYD, KJX, and WL analyzed the data; KJX and WL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Dong, Jy., Xia, Kj., Liang, W. et al. Ginsenoside Rb1 alleviates colitis in mice via activation of endoplasmic reticulum-resident E3 ubiquitin ligase Hrd1 signaling pathway. Acta Pharmacol Sin 42, 1461–1471 (2021). https://doi.org/10.1038/s41401-020-00561-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00561-9
Keywords
This article is cited by
-
Oral administration of Momordica charantia-derived extracellular vesicles alleviates ulcerative colitis through comprehensive renovation of the intestinal microenvironment
Journal of Nanobiotechnology (2025)
-
Ginseng exosomes modulate M1/M2 polarisation by activating autophagy and target IKK/IкB/NF-кB to alleviate inflammatory bowel disease
Journal of Nanobiotechnology (2025)
-
Ginsenoside Rb1 reduced ischemic stroke-induced apoptosis through endoplasmic reticulum stress-associated IRE1/TRAF2/JNK pathway
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Ginseng-derived nanoparticles alleviate inflammatory bowel disease via the TLR4/MAPK and p62/Nrf2/Keap1 pathways
Journal of Nanobiotechnology (2024)
-
Casting Light on the Janus-Faced HMG-CoA Reductase Degradation Protein 1: A Comprehensive Review of Its Dualistic Impact on Apoptosis in Various Diseases
Molecular Neurobiology (2024)