Abstract
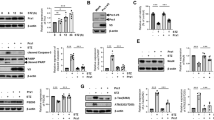
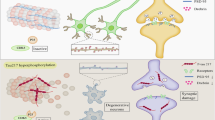
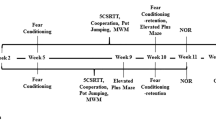
We previously reported that pseudoginsenoside-F11 (PF11), an ocotillol-type saponin, significantly ameliorated Alzheimer’s disease (AD)-associated cognitive defects in APP/PS1 and SAMP8 mice by inhibiting Aβ aggregation and tau hyperphosphorylation, suggesting a potential therapeutic effect of PF11 in the treatment of AD. In the present study we further evaluated the therapeutic effects of PF11 on relieving cognitive impairment in a rat model of sporadic AD (SAD). SAD was induced in rats by bilateral icv infusion of streptozotocin (STZ, 3 mg/kg). The rats were treated with PF11 (2, 4, 8 mg·kg−1·d−1, ig) or a positive control drug donepezil (5 mg·kg−1·d−1, ig) for 4 weeks. Their cognitive function was assessed in the nest building, Y-maze, and Morris water maze tests. We showed that STZ icv infusion significantly affected the cognitive function, tau phosphorylation, and insulin signaling pathway in the hippocampus. Furthermore, STZ icv infusion resulted in significant upregulation of the calpain I/cyclin-dependent protein kinase 5 (CDK5) signaling pathway in the hippocampus. Oral administration of PF11 dose-dependently ameliorated STZ-induced learning and memory defects. In addition, PF11 treatment markedly reduced the neuronal loss, protected the synapse structure, and modulated STZ-induced expression of tau phosphorylation by regulating the insulin signaling pathway and calpain I/CDK5 signaling pathway in the hippocampus. Donepezil treatment exerted similar beneficial effects in STZ-infused rats as the high dose of PF11 did. This study highlights the excellent therapeutic potential of PF11 in managing AD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement (N Y). 2018;4:195–214.
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat Rev Dis Prim. 2015;1:15056.
Rasheed NOA. Study of the possible protective effect of formoterol in streptozotocin-induced sporadic Alzheimer’s disease mouse model. Doctor of Philosophy degree. Cairo: Cairo University; 2019.
Chen Y, Liang Z, Blanchard J, Dai CL, Sun S, Lee MH, et al. A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Mol Neurobiol. 2013;47:711–25.
Ponce-Lopez T, Hong E, Abascal-Díaz M, Meneses A. Role of GSK3β and PP2A on regulation of tau phosphorylation in hippocampus and memory impairment in ICV-STZ animal model of Alzheimer’s disease. Adv Alzheimer Dis. 2017;06:13–31.
Du LL, Xie JZ, Cheng XS, Li XH, Kong FL, Jiang X, et al. Activation of sirtuin 1 attenuates cerebral ventricular streptozotocin-induced tau hyperphosphorylation and cognitive injuries in rat hippocampi. Age. 2014;36:613–23.
Gao C, Liu YZ, Jiang YH, Ding JM, Li L. Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3β pathway in streptozotocin‐induced Alzheimer rat model. Brain Pathol. 2014;24:261–9.
Grieb P. Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: in search of a relevant mechanism. Mol Neurobiol. 2016;53:1741–52.
Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci. 2015;9:204.
Lee MS, Kwon YT, Li MW, Peng JM, Friedlander RM, Tsai LS. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–4.
Kurbatskaya K, Phillips EC, Croft CL, Dentoni G, Hughes MM, Wade MA, et al. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer’s disease brain. Acta Neuropathol Commun. 2016;4:34.
Wang CM, Liu MY, Wang F, Wei MJ, Wang S, Wu CF, et al. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol Biochem Behav. 2013;106:57–67.
Liu YY, Zhang TY, Xue X, Liu DM, Zhang HT, Yuan LL, et al. Pseudoginsenoside-F11 attenuates cerebral ischemic injury by alleviating autophagic/lysosomal defects. CNS Neurosci Ther. 2017;23:567–79.
Wang X, Wang C, Wang J, Zhao S, Zhang K, Wang J, et al. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt signaling pathways. Neuropharmacology. 2014;79:642–56.
Li Z, Guo Y, Wu C, Li X, Wang J. Protective effects of pseudoginsenoside‐F11 on scopolamine‐induced memory impairment in mice and rats. J Pharm Pharmacol. 1999;51:435–40.
Zhang Z, Yang HL, Yang JY, Xie J, Xu JY, Liu C, et al. Pseudoginsenoside-F11 attenuates cognitive impairment by ameliorating oxidative stress and neuroinflammation in dgalactose-treated mice. Int Immunopharmacol. 2019;67:78–86.
Zhang Z, Yang JY, Liu C, Xie J, Qiu S, Yang X, et al. Pseudoginsenoside-F11 alleviates cognitive deficits and Alzheimer’s disease-type pathologies in SAMP8 mice. Pharmacol Res. 2019;139:512–23.
Kosaraju J, Gali CC, Khatwal RB, Dubala A, Chinni S, Holsinger RM, et al. Saxagliptin: a dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology. 2013;72:291–300.
Wang JY, Yang JY, Wang F, Fu SY, Hou Y, Jiang B, et al. Neuroprotective effect of pseudoginsenoside-f11 on a rat model of Parkinson’s disease induced by 6-hydroxydopamine. Evid Based Complement Altern Med. 2013;2013:152798.
Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, et al. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav. 2003;76:103–9.
Zhang N, Gordon ML. Clinical efficacy and safety of donepezil in the treatment of Alzheimer’s disease in Chinese patients. Clin Inter Aging. 2018;13:1963–70.
Agrawal R, Tyagi E, Shukla R, Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56:779–87.
Galasko D, Schmitt F, Thomas R, Jin S, Bennett D. Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc. 2005;11:446–53.
Roof R, Snyder B, Yabe Y, Zaleska M. Use of ethological rodent behavior to assess efficacy of potential drugs for Alzheimer’s disease. Proc Meas Behav. 2010;2010:389.
Galeano P, Martino Adami PV, Do Carmo S, Blanco E, Rotondaro C, Capani F, et al. Longitudinal analysis of the behavioral phenotype in a novel transgenic rat model of early stages of Alzheimer’s disease. Front Behav Neurosci. 2014;8:321.
Agrawal R, Mishra B, Tyagi E, Nath C, Shukla R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol Res. 2010;61:247–52.
Chen X, Wang N, Liu Y, Liu Y, Zhang T, Zhu L, et al. Yonkenafil: a novel phosphodiesterase type 5 inhibitor induces neuronal network potentiation by a cGMP-dependent Nogo-R axis in acute experimental stroke. Exp Neurol. 2014;261:267–77.
Llorens-Martín M, Fuster-Matanzo A, Teixeira CM, Jurado-Arjona J, Ulloa F, Defelipe J, et al. GSK-3 beta overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Mol Psychiatry. 2013;18:451–60.
Šimić G, Babić Leko M, Wray S, Harrington C, Delalle I, Jovanov-Milošević N, et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:6.
Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:167–79.
Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130.
Gao Y, Tan L, Yu JT, Tan L. Tau in Alzheimer’s disease: mechanisms and therapeutic strategies. Curr Alzheimer Res. 2018;15:283–300.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–38.
Akintola AA, van Heemst D. Insulin, aging, and the brain: mechanisms and implications. Front Endocrinol (Lausanne). 2015;6:13.
Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2006;9:309–17.
Kusakawa GI, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga SI. Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem. 2000;275:17166–72.
Patrick GN, Zukerberg L, Nikolic M, de La Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22.
Chow HM, Guo D, Zhou JC, Zhang GY, Li HF, Herrup K, et al. CDK5 activator protein p25 preferentially binds and activates GSK3β. Proc Natl Acad Sci U S A. 2014;111:E4887–95.
Zhang TY, Wu CF, Yang XW, Liu YY, Yang HL, Yuan LL, et al. Pseudoginsenoside-F11 protects against transient cerebral ischemia injury in rats involving repressing calcium overload. Neuroscience. 2019;411:86–104.
Laurent C, Buée L, Blum D. Tau and neuroinflammation: what impact for Alzheimer’s disease and tauopathies? Biomed J. 2018;41:21–33.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9.
Shin SJ, Jeong YO, Jeon SG, Kim S, Lee SK, Nam Y, et al. Jowiseungchungtang inhibits amyloid-beta aggregation and amyloid-beta-mediated pathology in 5XFAD mice. Int J Mol Sci. 2018;19:4026.
Grizzell JA, Patel S, Barreto GE, Echeverria V. Cotinine improves visual recognition memory and decreases cortical Tau phosphorylation in the Tg6799 mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:75–81.
Author information
Authors and Affiliations
Contributions
Designed research: LZ, XJH, JYY. Performed research: XJH, LZ, TSZ, XQL. Analyzed data: XJH, LZ. Wrote the manuscript: LZ. Revised the manuscript: XHC, JYY, CFW. All authors contributed substantially to this work and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhu, L., Hou, Xj., Che, Xh. et al. Pseudoginsenoside-F11 attenuates cognitive dysfunction and tau phosphorylation in sporadic Alzheimer’s disease rat model. Acta Pharmacol Sin 42, 1401–1408 (2021). https://doi.org/10.1038/s41401-020-00562-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00562-8
Keywords
This article is cited by
-
Potential of phytochemicals in the treatment of Alzheimer disease by modulating lysosomal dysfunction: a systematic review
Chinese Medicine (2025)
-
Sex-Dependent Changes in the Gene Expression of UPR-Associated Calcium-Binding Proteins in the STZ–Induced Model of Alzheimer’s Disease
Molecular Neurobiology (2025)
-
The identification of key genes and pathways in polycystic ovary syndrome by bioinformatics analysis of next-generation sequencing data
Middle East Fertility Society Journal (2024)
-
Abnormal protein post-translational modifications induces aggregation and abnormal deposition of protein, mediating neurodegenerative diseases
Cell & Bioscience (2024)
-
MST1 selective inhibitor Xmu-mp-1 ameliorates neuropathological changes in a rat model of sporadic Alzheimer’s Disease by modulating Hippo-Wnt signaling crosstalk
Apoptosis (2024)