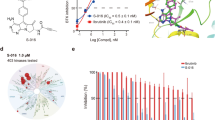
Abstract
Rheumatoid arthritis (RA) is characterized by joint leukocyte infiltration, synovial inflammation and bone damage result from osteoclastogenesis. Bruton’s tyrosine kinase (BTK) is a key regulator of B cell receptor (BCR) and Fc gamma receptor (FcγR) signaling involved in the pathobiology of RA and other autoimmune disorders. SOMCL-17-016 is a potent and selective tricyclic BTK inhibitor, structurally distinct from other known BTK inhibitors. In present study we investigated the therapeutic efficacy of SOMCL-17-016 in a mouse collagen-induced arthritis (CIA) model and underlying mechanisms. CIA mice were administered SOMCL-17-016 (6.25, 12.5, 25 mg·kg−1·d−1, ig), or ibrutinib (25 mg·kg−1·d−1, ig) or acalabrutinib (25 mg·kg−1·d−1, ig) for 15 days. We showed that oral administration of SOMCL-17-016 dose-dependently ameliorated arthritis severity and bone damage in CIA mice; it displayed a higher in vivo efficacy than ibrutinib and acalabrutinib at the corresponding dosage. We found that SOMCL-17-016 administration dose-dependently inhibited anti-IgM-induced proliferation and activation of B cells from CIA mice, and significantly decreased anti-IgM/anti-CD40-stimulated RANKL expression in memory B cells from RA patients. In RANKL/M-CSF-stimulated RAW264.7 cells, SOMCL-17-016 prevented osteoclast differentiation and abolished RANK-BTK-PLCγ2-NFATc1 signaling. In summary, this study demonstrates that SOMCL-17-016 presents distinguished therapeutic effects in the CIA model. SOMCL-17-016 exerts a dual inhibition of B cell function and osteoclastogenesis, suggesting that it to be a promising drug candidate for RA treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Managed Care. 2012;18:S295–302.
Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–61.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Derksen V, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol. 2017;39:437–46.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
Jia XY, Chang Y, Wei F, Dai X, Wu YJ, Sun XJ, et al. CP-25 reverses prostaglandin E4 receptor desensitization-induced fibroblast-like synoviocyte dysfunction via the G protein-coupled receptor kinase 2 in autoimmune arthritis. Acta Pharmacol Sin. 2019;40:1029–39.
Han L, Zhang XZ, Wang C, Tang XY, Zhu Y, Cai XY, et al. IgD-Fc-Ig fusion protein, a new biological agent, inhibits T cell function in CIA rats by inhibiting IgD-IgDR-Lck-NF-kappaB signaling pathways. Acta Pharmacol Sin. 2020;41:800–12.
Steffen U, Schett G, Bozec A. How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol. 2019;10:1483.
Seeling M, Hillenhoff U, David JP, Schett G, Tuckermann J, Lux A, et al. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc Natl Acad Sci USA. 2013;110:10729–34.
Engdahl C, Bang H, Dietel K, Lang SC, Harre U, Schett G. Periarticular bone loss in arthritis is induced by autoantibodies against citrullinated vimentin. J Bone Miner Res. 2017;32:1681–91.
Komine M, Kukita A, Kukita T, Ogata Y, Hotokebuchi T, Kohashi O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001;28:474–83.
Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275:4858–64.
Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;283:11526–34.
Ariza Y, Murata M, Ueda Y, Yoshizawa T. Bruton’s tyrosine kinase (Btk) inhibitor tirabrutinib suppresses osteoclastic bone resorption. Bone Rep. 2019;10:100201.
Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806.
Shinohara M, Chang BY, Buggy JJ, Nagai Y, Kodama T, Asahara H, et al. The orally available Btk inhibitor ibrutinib (PCI-32765) protects against osteoclast-mediated bone loss. Bone. 2014;60:8–15.
Satterthwaite AB, Witte ON. The role of Bruton’s tyrosine kinase in B-cell development and function: a genetic perspective. Immunol Rev. 2000;175:120–7.
Tomlinson MG, Kane LP, Su J, Kadlecek TA, Mollenauer MN, Weiss A. Expression and function of Tec, Itk, and Btk in lymphocytes: evidence for a unique role for Tec. Mol Cell Biol. 2004;24:2455–66.
Musilova K, Mraz M. MicroRNAs in B-cell lymphomas: how a complex biology gets more complex. Leukemia. 2015;29:1004–17.
Ota Y, Niiro H, Ota S, Ueki N, Tsuzuki H, Nakayama T, et al. Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2016;18:67.
Hamilton JA, Tak PP. The dynamics of macrophage lineage populations in inflammatory and autoimmune diseases. Arthritis Rheum. 2009;60:1210–21.
Pan Z, Scheerens H, Li SJ, Schultz BE, Sprengeler PA, Burrill LC, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007;2:58–61.
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–80.
Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–52.
Yu H, Truong H, Mitchell SA, Liclican A, Gosink JJ, Li W, et al. Homogeneous BTK occupancy assay for pharmacodynamic assessment of tirabrutinib (GS-4059/ONO-4059) target engagement. SLAS Discov. 2018;23:919–29.
Evans EK, Tester R, Aslanian S, Karp R, Sheets M, Labenski MT, et al. Inhibition of Btk with CC-292 provides early pharmacodynamic assessment of activity in mice and humans. J Pharmacol Exp Ther. 2013;346:219–28.
Zhou R, Tang W, Ren YX, He PL, Zhang F, Shi LP, et al. (5R)-5-hydroxytriptolide attenuated collagen-induced arthritis in DBA/1 mice via suppressing interferon-gamma production and its related signaling. J Pharmacol Exp Ther. 2006;318:35–44.
Schramm C, Kriegsmann J, Protschka M, Huber S, Hansen T, Schmitt E, et al. Susceptibility to collagen-induced arthritis is modulated by TGFbeta responsiveness of T cells. Arthritis Res Ther. 2004;6:R114–9.
Chang Y, Wu Y, Wang D, Wei W, Qin Q, Xie G, et al. Therapeutic effects of TACI-Ig on rats with adjuvant-induced arthritis via attenuating inflammatory responses. Rheumatology (Oxford). 2011;50:862–70.
Lin ZM, Yang XQ, Zhu FH, He SJ, Tang W, Zuo JP. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci Rep. 2016;6:38115.
Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–55.
He SJ, Lin ZM, Wu YW, Bai BX, Yang XQ, He PL, et al. Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZB×NZW F1 mice via interference with TLR-mediated APC response. Acta Pharmacol Sin. 2014;35:219–29.
Hou LF, He SJ, Wang JX, Yang Y, Zhu FH, Zhou Y, et al. SM934, a water-soluble derivative of arteminisin, exerts immunosuppressive functions in vitro and in vivo. Int Immunopharmacol. 2009;9:1509–17.
Sato K, Takayanagi HOsteoclasts. rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol. 2006;18:419–26.
Di Paolo JA, Huang T, Balazs M, Barbosa J, Barck KH, Bravo BJ, et al. Specific Btk inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat Chem Biol. 2011;7:41–50.
Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, et al. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003–12.
Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–8.
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69.
Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011;70:2022–8.
Acknowledgements
This work was supported by the Personalized Medicines—Molecular Signature-based “Drug Discovery and Development,” Strategic Priority Research Program of the Chinese Academy of Sciences (grant No. XDA12020369); the National Natural Science Foundation of China (NSFC) (grant No. 81903882); and the National Science and Technology Major Project “New Drug Creation and Manufacturing Program,” China (grant No. 2018ZX09711002-014-001).
Author information
Authors and Affiliations
Contributions
YTL, HHD, AZ, SJH, and JPZ contributed to the conception and design of this study; YTL and ZML completed the main part of the experiment; QW, LC, XQY and FHZ participated in the in vivo experiment; HHD, SSL, YTH, SQC, FMY participated in the in vitro experiment; ZLS contributed to the synthesis of the compound; AZ, SJH, JPZ, JD, MYG and HX contributed to drafting the paper and revising it critically for important intellectual content; and all authors contributed to this paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Liu, Yt., Ding, Hh., Lin, Zm. et al. A novel tricyclic BTK inhibitor suppresses B cell responses and osteoclastic bone erosion in rheumatoid arthritis. Acta Pharmacol Sin 42, 1653–1664 (2021). https://doi.org/10.1038/s41401-020-00578-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00578-0
Keywords
This article is cited by
-
The inactive X chromosome accumulates widespread epigenetic variability with age
Clinical Epigenetics (2023)