Abstract
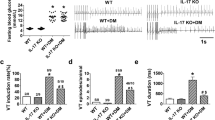
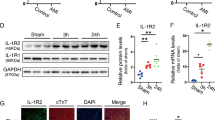
Interleukin-17 (IL-17), also called IL-17A, is an important regulator of cardiac diseases, but its role in calcium-related cardiac dysfunction remains to be explored. Thus, we investigated the influence of IL-17 on calcium handling process and its contribution to the development of heart failure. Mice were subjected to transaortic constriction (TAC) to induce heart failure. In these mice, the levels of IL-17 in the plasma and cardiac tissue were significantly increased compared with the sham group. In 77 heart failure patients, the plasma level of IL-17 was significantly higher than 49 non-failing subjects, and was negatively correlated with cardiac ejection fraction and fractional shortening. In IL-17 knockout mice, the shortening of isolated ventricular myocytes was increased compared with that in wild-type mice, which was accompanied by significantly increased amplitude of calcium transient and the upregulation of SERCA2a and Cav1.2. In cultured neonatal cardiac myocytes, treatment of with IL-17 (0.1, 1 ng/mL) concentration-dependently suppressed the amplitude of calcium transient and reduced the expression of SERCA2a and Cav1.2. Furthermore, IL-17 treatment increased the expression of the NF-κB subunits p50 and p65, whereas knockdown of p50 reversed the inhibitory effects of IL-17 on SERCA2a and Cav1.2 expression. In mice with TAC-induced mouse heart, IL-17 knockout restored the expression of SERCA2a and Cav1.2, increased the amplitude of calcium transient and cell shortening, and in turn improved cardiac function. In addition, IL-17 knockout attenuated cardiac hypertrophy with inhibition of calcium-related signaling pathway. In conclusion, upregulation of IL-17 impairs cardiac function through NF-κB-mediated disturbance of calcium handling and cardiac remodeling. Inhibition of IL-17 represents a potential therapeutic strategy for the treatment of heart failure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bartekova M, Radosinska J, Jelemensky M, Dhalla NS. Role of cytokines and inflammation in heart function during health and disease. Heart Fail Rev. 2018;23:733–58.
Schumacher SM, Naga Prasad SV. Tumor necrosis factor-alpha in heart failure: an updated review. Curr Cardiol Rep. 2018;20:117.
Bozkurt B, Torre-Amione G, Warren MS, Whitmore J, Soran OZ, Feldman AM, et al. Results of targeted anti-tumor necrosis factor therapy with etanercept (ENBREL) in patients with advanced heart failure. Circulation. 2001;103:1044–7.
Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205.
Rahm AK, Lugenbiel P, Schweizer PA, Katus HA, Thomas D. Role of ion channels in heart failure and channelopathies. Biophys Rev. 2018;10:1097–106.
Peana D, Domeier TL. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr Opin Pharmacol. 2017;33:17–26.
Tsai CT, Wu CK, Lee JK, Chang SN, Kuo YM, Wang YC, et al. TNF-alpha down-regulates sarcoplasmic reticulum Ca2+ ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-kappaB to promoter response element. Cardiovasc Res. 2015;105:318–29.
Hobai IA, Morse JC, Siwik DA, Colucci WS. Lipopolysaccharide and cytokines inhibit rat cardiomyocyte contractility in vitro. J Surg Res. 2015;193:888–901.
Rahmati Z, Amirzargar AA, Saadati S, Rahmani F, Mahmoudi MJ, Rahnemoon Z, et al. Association of levels of interleukin 17 and T-helper 17 count with symptom severity and etiology of chronic heart failure: a case-control study. Croat Med J. 2018;59:139–48.
Zhou SF, Yuan J, Liao MY, Xia N, Tang TT, Li JJ, et al. IL-17A promotes ventricular remodeling after myocardial infarction. J Mol Med (Berl). 2014;92:1105–16.
Zhang Y, Zhang YY, Li TT, Wang J, Jiang Y, Zhao Y, et al. Ablation of interleukin-17 alleviated cardiac interstitial fibrosis and improved cardiac function via inhibiting long non-coding RNA-AK081284 in diabetic mice. J Mol Cell Cardiol. 2018;115:64–72.
Zhang Y, Jia S, Gao T, Zhang R, Liu Z, Wang Y. Dexmedetomidine mitigate acute lung injury by inhibiting IL-17-induced inflammatory reaction. Immunobiology. 2018;223:32–7.
Pan Z, Sun X, Shan H, Wang N, Wang J, Ren J, et al. MicroRNA-101 inhibited postinfarct cardiac fibrosis and improved left ventricular compliance via the FBJ osteosarcoma oncogene/transforming growth factor-beta1 pathway. Circulation. 2012;126:840–50.
El Khoury N, Mathieu S, Fiset C. Interleukin-1beta reduces L-type Ca2+ current through protein kinase C activation in mouse heart. J Biol Chem. 2014;289:21896–908.
Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, et al. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–44.
Nelson BR, Makarewich CA, Anderson DM, Winders BR, Troupes CD, Wu F, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–5.
Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–62.
Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology. 2005;129:1518–32.
Duncan DJ, Yang Z, Hopkins PM, Steele DS, Harrison SM. TNF-alpha and IL-1beta increase Ca2+ leak from the sarcoplasmic reticulum and susceptibility to arrhythmia in rat ventricular myocytes. Cell Calcium. 2010;47:378–86.
Greensmith DJ, Nirmalan M. The effects of tumor necrosis factor-alpha on systolic and diastolic function in rat ventricular myocytes. Physiol Rep. 2013;1:e00093.
Cao CM, Xia Q, Bruce IC, Shen YL, Ye ZG, Lin GH, et al. Influence of interleukin-2 on Ca2+ handling in rat ventricular myocytes. J Mol Cell Cardiol. 2003;35:1491–503.
Cao CM, Xia Q, Bruce IC, Zhang X, Fu C, Chen JZ. Interleukin-2 increases activity of sarcoplasmic reticulum Ca2+-ATPase, but decreases its sensitivity to calcium in rat cardiomyocytes. J Pharmacol Exp Ther. 2003;306:572–80.
Krown KA, Yasui K, Brooker MJ, Dubin AE, Nguyen C, Harris GL, et al. TNF alpha receptor expression in rat cardiac myocytes: TNF alpha inhibition of L-type Ca2+ current and Ca2+ transients. FEBS Lett. 1995;376:24–30.
Amatya N, Garg AV, Gaffen SL. IL-17 signaling: the Yin and the Yang. Trends Immunol. 2017;38:310–22.
Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J Clin Invest. 1995;96:1093–9.
Zhao L, Cheng G, Jin R, Afzal MR, Samanta A, Xuan YT, et al. Deletion of interleukin-6 attenuates pressure overload-induced left ventricular hypertrophy and dysfunction. Circ Res. 2016;118:1918–29.
Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–6.
Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–406.
Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca2+-dependent transgenic model of cardiac hypertrophy: a role for protein kinase Calpha. Circulation. 2001;103:140–7.
Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, et al. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122:280–90.
Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S, Weber C. Immunotherapy for cardiovascular disease. Eur Heart J. 2019;40:3937–46.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–40.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–99.
Acknowledgements
This work was supported by National Key R&D Program of China (2017YFC1307404 to ZWP), National Natural Science Foundation of China (81730012 to BFY and 81870295 to ZWP), Funds for Distinguished Young Scholars of Heilongjiang Province (to ZP), Heilongjiang Touyan Innovation Team Program (to BFY) and Yu Weihan Excellent Youth Foundation of Harbin Medical University (001000004 to ZWP).
Author information
Authors and Affiliations
Contributions
GLX and DSL performed experiments, analyzed data, and prepared the paper. ZYW, YL, JMY, CZL, XDL, JDM, MMZ, YJL and YL helped perform experiments and collect data. BFY and ZWP designed the project, oversaw the experiments and prepared the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Xue, Gl., Li, Ds., Wang, Zy. et al. Interleukin-17 upregulation participates in the pathogenesis of heart failure in mice via NF-κB-dependent suppression of SERCA2a and Cav1.2 expression. Acta Pharmacol Sin 42, 1780–1789 (2021). https://doi.org/10.1038/s41401-020-00580-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00580-6
Keywords
This article is cited by
-
The gut microbiota metabolite trimethylamine N-oxide promotes cardiac hypertrophy by activating the autophagic degradation of SERCA2a
Communications Biology (2025)
-
SERCA2a dysfunction in the pathophysiology of heart failure with preserved ejection fraction: a direct role is yet to be established
Heart Failure Reviews (2025)
-
Immunomodulation and immunopharmacology in heart failure
Nature Reviews Cardiology (2024)
-
LncRNA CCRR maintains Ca2+ homeostasis against myocardial infarction through the FTO-SERCA2a pathway
Science China Life Sciences (2024)
-
NLRP3 Inflammasome: a Novel Insight into Heart Failure
Journal of Cardiovascular Translational Research (2023)