Abstract
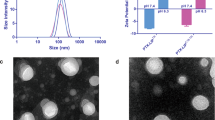
Lung cancer is one of the leading causes of cancer-related death worldwide. Various therapeutic failed in the effective treatment of the lung cancer due to their limited accumulation and exposure in tumors. In order to promote the chemotherapeutics delivery to lung tumor, we introduced chitosan oligosaccharide (CSO) modification on the liposomes. CSO conjugated Pluronic P123 polymers with different CSO grafting amounts, called as CP50 and CP20, were synthesized and used to prepare CSO modified liposomes (CP50-LSs and CP20-LSs). CP50-LSs and CP20-LSs displayed significantly enhanced cellular uptake in A549 cells in vitro as well as superior tumor accumulation in vivo compared with non-CSO modified liposomes (P-LSs). This phenomenon was related to the increased affinity between CSO modified liposomes and tumor cells following massive adsorption of collagen, which was highly expressed in lung tumors. In the A549 tumor-bearing mouse model, intravenous injection of paclitaxel (PTX)-loaded CP50-LSs every 3 days for 21 days resulted in optimal antitumor therapeutic performance with an inhibition rate of 86.4%. These results reveal that CSO modification provides promising applicability for nanomedicine design in the lung cancer treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wong MCS, Lao XQ, Ho KF, Goggins WB, Tse SLA. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7:14300–8.
Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5:288–300.
Zhang Y, Yang J, Ding M, Li L, Lu Z, Zhang Q, et al. Tumor-penetration and antitumor efficacy of cetuximab are enhanced by co-administered iRGD in a murine model of human NSCLC. Oncol Lett. 2016;12:3241–9.
Liang XJ, Chen C, Zhao Y, Wang PC. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol Biol. 2010;596:467–88.
Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141:320–7.
Yu T, Hubbard D, Ray A, Ghandehari H. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J Control Release. 2012;163:46–54.
Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–27.
Lim YH, Tiemann KM, Hunstad DA, Elsabahy M, Wooley KL. Polymeric nanoparticles in development for treatment of pulmonary infectious diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:842–71.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–87.
Agnoletti M, Rodriguez-Rodriguez C, Klodzinska SN, Esposito TVF, Saatchi K, Morck Nielsen H, et al. Monosized polymeric microspheres designed for passive lung targeting: biodistribution and pharmacokinetics after intravenous administration. ACS Nano. 2020;14:6693–706.
Au KM, Min Y, Tian X, Zhang L, Perello V, Caster JM, et al. Improving cancer chemoradiotherapy treatment by dual controlled release of wortmannin and docetaxel in polymeric nanoparticles. ACS Nano. 2015;9:8976–96.
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93.
Merkel OM, Beyerle A, Librizzi D, Pfestroff A, Behr TM, Sproat B, et al. Nonviral siRNA delivery to the lung: investigation of PEG-PEI polyplexes and their in vivo performance. Mol Pharmacol. 2009;6:1246–60.
Jin H, Xu CX, Kim HW, Chung YS, Shin JY, Chang SH, et al. Urocanic acid-modified chitosan-mediated PTEN delivery via aerosol suppressed lung tumorigenesis in K-ras(LA1) mice. Cancer Gene Ther. 2008;15:275–83.
Nascimento AV, Singh A, Bousbaa H, Ferreira D, Sarmento B, Amiji MM. Mad2 checkpoint gene silencing using epidermal growth factor receptor-targeted chitosan nanoparticles in non-small cell lung cancer model. Mol Pharmacol. 2014;11:3515–27.
Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, et al. Biological activity of chitosan: ultrastructural study. Biomaterials 1988;9:247–52.
Muzzarelli RA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell Mol Life Sci. 1997;53:131–40.
George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan-a review. J Control Release. 2006;114:1–14.
Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274:1–33.
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017;9:53–78.
Pavinatto FJ, Pavinatto A, Caseli L, dos Santos DS, Nobre TM, Zaniquelli MED, et al. Interaction of chitosan with cell membrane models at the air-water interface. Biomacromolecules 2007;8:1633–40.
Huang M, Ma Z, Khor E, Lim LY. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm Res. 2002;19:1488–94.
Vijayakurup V, Thulasidasan AT, Shankar GM, Retnakumari AP, Nandan CD, Somaraj J, et al. Chitosan encapsulation enhances the bioavailability and tissue retention of curcumin and improves its efficacy in preventing B[a]P-induced lung carcinogenesis. Cancer Prev Res (Philos). 2019;12:225–36.
Jia LJ, Li ZY, Zhang DR, Zhang Q, Shen JY, Guo HJ, et al. Redox-responsive catiomer based on PEG-ss-chitosan oligosaccharide-ss-polyethylenimine copolymer for effective gene delivery. Polym Chem-UK. 2013;4:156–65.
Pillai CKS, Paul W, Sharma CP. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci. 2009;34:641–78.
Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97.
Abrica-Gonzalez P, Zamora-Justo JA, Sotelo-Lopez A, Vazquez-Martinez GR, Balderas-Lopez JA, Munoz-Diosdado A, et al. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res Lett. 2019;14:258–71.
Hu FQ, Chen WW, Zhao MD, Yuan H, Du YZ. Effective antitumor gene therapy delivered by polyethylenimine-conjugated stearic acid-g-chitosan oligosaccharide micelles. Gene Ther. 2013;20:597–606.
Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–33.
Ohashi T, Yoshimasu T, Oura S, Kokawa Y, Kawago M, Hirai Y, et al. Class III beta-tubulin expression in non-small cell lung cancer: a predictive factor for paclitaxel response. Anticancer Res. 2015;35:2669–74.
Sun W, Jiang Y, He F. Extraction and proteome analysis of liver tissue interstitial fluid. Methods Mol Biol. 2011;728:247–57.
De Jaeghere F, Allemann E, Feijen J, Kissel T, Doelker E, Gurny R. Cellular uptake of PEO surface-modified nanoparticles: Evaluation of nanoparticles made of PLA: PEO diblock and triblock copolymers. J Drug Target. 2000;8:143–53.
Suk JS, Xu QG, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Zhu YQ, Chen C, Cao ZY, Shen S, Li LS, Li DD, et al. On-demand PEGylation and dePEGylation of PLA-based nanocarriers via amphiphilic mPEG-TK-Ce6 for nanoenabled cancer chemotherapy. Theranostics 2019;9:8312–20.
Soenen SJ, Manshian BB, Abdelmonem AM, Montenegro JM, Tan S, Balcaen L, et al. The cellular interactions of PEGylated gold nanoparticles: effect of PEGylation on cellular uptake and cytotoxicity. Part Part Syst Char. 2014;31:794–800.
Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–37.
Monopoli MP, Aberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7:779–86.
Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. J Biomed Mater Res. 2000;49:435–47.
Schottler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, et al. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–7.
Wang XY, Wang MZ, Lei R, Zhu SF, Zhao YL, Chen CY. Chiral surface of nanoparticles determines the orientation of adsorbed transferrin and its interaction with receptors. ACS Nano. 2017;11:4606–16.
Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Saf. 2007;6:609–21.
Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–8.
Wang F, Porter M, Konstantopoulos A, Zhang P, Cui H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J Control Release. 2017;267:100–18.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81973250), the National Science and Technology Major Project (2018ZX09721002-003) and the National Key Research and Development Program of China (NBHY-2017-J1-3).
Author information
Authors and Affiliations
Contributions
RW, XXZ, and YG designed the project; YQM and JJZ prepared the liposomes. YQM, MSC, and LMG designed and performed all experiments. All authors analyzed and discussed the data. YQM, MSC, XZ, and XXZ wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Miao, Yq., Chen, Ms., Zhou, X. et al. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharmacol Sin 42, 1714–1722 (2021). https://doi.org/10.1038/s41401-020-00594-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00594-0
Keywords
This article is cited by
-
Breathing new life nanomedicines for pulmonary drug delivery: targeting approaches, experimental models, and regulatory aspects
Beni-Suef University Journal of Basic and Applied Sciences (2025)
-
Construction of Chitosan Oligosaccharide-Coated Nanostructured Lipid Carriers for the Sustained Release of Strontium Ranelate
Tissue Engineering and Regenerative Medicine (2025)
-
Regulating protein corona on nanovesicles by glycosylated polyhydroxy polymer modification for efficient drug delivery
Nature Communications (2024)