Abstract
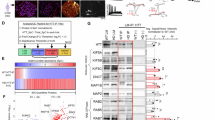
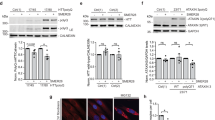
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by toxic aggregates of mutant huntingtin protein (mHTT) in the brain. Decreasing mHTT is a potential strategy for therapeutic purpose of HD. Valosin-containing protein (VCP/p97) is a crucial regulator of proteostasis, which regulates the degradation of damaged protein through proteasome and autophagy pathway. Since VCP has been implicated in pathogenesis of HD as well as other neurodegenerative diseases, small molecules that specifically regulate the activity of VCP may be of therapeutic benefits for HD patients. In this study we established a high-throughput screening biochemical assay for VCP ATPase activity measurement and identified gossypol, a clinical approved drug in China, as a novel modulator of VCP. Gossypol acetate dose-dependently inhibited the enzymatic activity of VCP in vitro with IC50 of 6.53±0.6 μM. We further demonstrated that gossypol directly bound to the interface between the N and D1 domains of VCP. Gossypol acetate treatment not only lowered mHTT levels and rescued HD-relevant phenotypes in HD patient iPS-derived Q47 striatal neurons and HD knock-in mouse striatal cells, but also improved motor function deficits in both Drosophila and mouse HD models. Taken together, gossypol acetate acted through a gain-of-function way to induce the formation of VCP-LC3-mHTT ternary complex, triggering autophagic degradation of mHTT. This study reveals a new strategy for treatment of HD and raises the possibility that an existing drug can be repurposed as a new treatment of neurodegenerative diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–6.
Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, et al. Spinocerebellar ataxia 3 and machado-joseph disease: clinical, molecular, and neuropathoiogicai features. Ann Neurol. 1996;39:490–9.
Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36.
Iwai A, Masliah E, Yoshimoto M. The precursor protein of non-Ap component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–75.
Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–73.
Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60.
Yu S, Liang Y, Palacino J, Difiglia M, Lu B. Drugging unconventional targets: insights from Huntington’s disease. Trends Pharmacol Sci. 2014;35:53–62.
Tsvetkov AS, Arrasate M, Barmada S, Ando DM, Sharma P, Shaby BA, et al. Proteostasis of polyglutamine varies among neurons and predicts neurodegeneration. Nat Chem Biol. 2013;9:586–92.
A Kakizuka, VCP, a major ATPase in the cells, as a novel target for currently incurable disorders. Innovative Medicine: Basic Research and Development. Tokyo: Springer; (2015).
Meyer H, Weihl CC. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014;127:3877–83.
Bug M, Meyer H. Expanding into new markets-VCP/p97 in endocytosis and autophagy. J Struct Biol. 2012;179:78–82.
Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, et al. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–27.
Van den BJ, Meyer H. VCP/p97-mediated unfolding as a principle in protein homeostasis and signaling. Mol Cell. 2018;69:182–94.
Hirabayashi M, Inoue K, Tanaka K, Nakadate K, Ohsawa Y, Kamei Y, et al. VCP/p97 in abnormal protein aggregates, cytoplasmic vacuoles, and cell death, phenotypes relevant to neurodegeneration. Cell Death Differ. 2001;8:977–984.
Fujita K, et al. A functional deficiency of TERA/VCP/p97 contributes to impaired DNA damage repair in multiple polyglutamine diseases. Nat Commun. 2013;4:1816.
Yang H, Li JJ, Liu S, Zhao J, Jiang YJ, Song AX, et al. Aggregation of polyglutamine-expanded ataxin-3 sequesters its specific interacting partners into inclusions: implication in a loss-of-function pathology. Sci Rep. 2014;4:6410.
Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176:11–42.
Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–34.
Sarkar S, Rubinsztein DC. Huntington’s disease: degradation of mutant huntingtin by autophagy. FEBS J. 2008;275:4263–70.
Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30.
Jimenez-Sanchez M, Thomson F, Zavodszky E, Rubinsztein DC. Autophagy and polyglutamine diseases. Prog Neurobiol. 2012;97:67–82.
He H, Dang Y, Dai F, Guo Z, Wu J, She X, et al. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem. 2003;278:29278–87.
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45.
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–11.
Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14.
Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, et al. VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease. Nat Commun. 2016;7:12646.
Xie H, Yin J, Shah MH, Menefee ME, Bible KC, Reidy-Lagunes D, et al. A phase II study of the orally administered negative enantiomer of gossypol (AT-101), a BH3 mimetic, in patients with advanced adrenal cortical carcinoma. Invest New Drugs. 2019;37:755–62.
Magnaghi P, D’Alessio R, Valsasina B, Avanzi N, Rizzi S, Asa D, et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat Chem Biol. 2013;9:548–56.
Y Yao, X Cui, I Al-Ramahi, X Sun, B Li, J Hou, et al. A striatal-enriched intronic GPCR modulates huntingtin levels and toxicity. Elife. 2015;4:e05449.
Baldo B, Paganetti P, Grueninger S. TR-FRET-based duplex immunoassay reveals an inverse correlation of soluble and aggregated mutant huntingtin in Huntington’s Disease. Chem Biol. 2012;19:264–75.
Kwakye GF, Li D, Bowman AB. Novel high-throughput assay to assess cellular manganese levels in a striatal cell line model of Huntington’s disease confirms a deficit in manganese accumulation. Neurotoxicology. 2011;3:630–9.
Almeida B, Abreu IA, Matos CA, Fraga JS, Fernandes S, Macedo MG, et al. SUMOylation of the brain-predominant Ataxin-3 isoform modulates its interaction with p97. Biochim Biophys Acta 2015;1852:1950–9.
Al-Ramahi I, Lam YC, Chen HK, de Gouyon B, Zhang M, Pérez AM, et al. CHIP protects from the neurotoxicity of expanded and wild-type Ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem. 2006;281:26714–24.
Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–15.
Huai Q, Kim HY, Liu Y, Zhao Y, Mondragon A, Liu JO, et al. Crystal structure of calcineurin-cyclophilin-cyclosporin shows common but distinct recognition of immunophilin-drug complexes. Proc Natl Acad Sci USA. 2002;99:12037–42.
Chapman E, Maksim N, de la Cruz F, La JJ. Clai, inhibitors of the AAA+ chaperon p97. Molecules. 2015;20:3027–49.
Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;1:117–23.
Lu B, Palacino J. A novel human embryonic stem cell-derived Huntington’s disease neuronal model exhibits mutant huntingtin (mHTT) aggregates and soluble mHTT-dependent neurodegeneration. FASEB J. 2013;27:1820–9.
Tabrizi SJ, Leavitt BR, Landwehrmeyer GB, Wild EJ, Saft C, Barker RA, et al. Targeting huntingtin expression in patients with Huntington’s disease. N Engl J Med. 2019;380:2307–16.
Park J, Shim JK, Kang JH, Choi J, Chang JH, Kim SY, et al. Regulation of bioenergetics through dual inhibition of aldehyde dehydrogenase and mitochondrial complex I suppresses glioblastoma tumorspheres. Neuro Oncol. 2018;20:954–65.
Li J, Wang C, Wang Z, Zhu C, Li J, Sha T, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds. Nature. 2019;575:203–9.
Chang L, Bertelsen EB, Wisén S, Larsen EM, Zuiderweg ER, Gestwicki JE. High-throughput screen for small molecules that modulate the ATPase activity of the molecular chaperone DnaK. Anal Biochem. 2008;372:167–76.
Weiss A, Abramowski D, Bibel M, Bodner R, Chopra V, DiFiglia M, et al. Single-step detection of mutant huntingtin in animal and human tissues:A bioassay for Huntington’s disease. Anal Biochem. 2009;395:8–15.
Hickey MA, Zhu C, Medvedeva V, Lerner RP, Patassini S, Franich NR, et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol Neurodegener. 2012;7:12.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31270830 and 21572038 to YJD; 81870990 to BXL;81625022, 91853205, 81821005 to CL; 31970748 to YHF), the Science and Technology Commission of Shanghai Municipality (18431907100 and 19XD1404700 to C.L.), the Development Fund for Shanghai Talents, Fund of State Key Laboratory of Bioorganic and Natural Products Chemistry, and Fund of State Key Laboratory of Drug Research and Chinese Academy of Science (SIMM1601KF-08). The authors acknowledge instructive advice and support from Prof. Fang Huang from Fudan University and her student Yu-fang Yang, Prof. Jun O. Liu from the Johns Hopkins University and Prof. Jian-zhong Yu from the University of Kansas.
Author information
Authors and Affiliations
Contributions
XJL performed the ATPase assay, PTS assay, pull-down assay, Western blot analysis, mouse behavioral experiments and the corresponding data analysis, and wrote the manuscript. YYZ contributed to manuscript writing and modification and was involved in the mouse behavioral experiments. YHF contributed to the HTRF assay, neuronal loss, and apoptosis measurement. HZ contributed to simulating the interaction model of GA and VCP. HXL completed Drosophila model behavior experiments. QFL was involved in performing the Western blot analysis. HLL was involved in the mouse behavioral experiments. RKT conducted the HTS. CXJ performed partial proteolysis assays and ITC experiments. WJ and ZXL were involved in protein expression and purification, ITC, and mouse behavioral experiments. CL conceived and supervised the simulation of the interaction model. BXL conceived and supervised the HD cell and animal experiments. YJD conceived and supervised the project and modified the manuscripts.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Xj., Zhang, Yy., Fu, Yh. et al. Gossypol, a novel modulator of VCP, induces autophagic degradation of mutant huntingtin by promoting the formation of VCP/p97-LC3-mHTT complex. Acta Pharmacol Sin 42, 1556–1566 (2021). https://doi.org/10.1038/s41401-020-00605-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-00605-0
Keywords
This article is cited by
-
Tau accumulation is cleared by the induced expression of VCP via autophagy
Acta Neuropathologica (2024)
-
Valosin containing protein (VCP): initiator, modifier, and potential drug target for neurodegenerative diseases
Molecular Neurodegeneration (2023)
-
Acetaldehyde Induces Cytotoxicity via Triggering Mitochondrial Dysfunction and Overactive Mitophagy
Molecular Neurobiology (2022)