Abstract
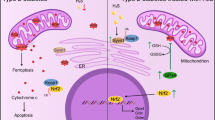
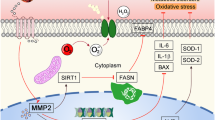
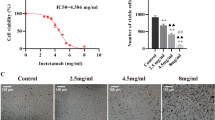
Gastrodin (GAS) is the main bioactive component of Tianma, a traditional Chinese medicine widely used to treat neurological disorders as well as cardio- and cerebrovascular diseases. In the present study, the protective effects of GAS on H9c2 cells against ischemia–reperfusion (IR)-like injury were found to be related to decreasing of oxidative stress. Furthermore, GAS could protect H9c2 cells against oxidative injury induced by H2O2. Pretreatment of GAS at 20, 50, and 100 μM for 4 h significantly ameliorated the decrease in cell viability and increase in apoptosis of H9c2 cells treated with 400 μM H2O2 for 3 h. Furthermore, we showed that H2O2 treatment induced fragmentation of mitochondria and significant reduction in networks, footprint, and tubular length of mitochondria; H2O2 treatment strongly inhibited mitochondrial respiration; H2O2 treatment induced a decrease in the expression of mitochondrial fusion factors Mfn2 and Opa1, and increase in the expression of mitochondrial fission factor Fis1. All these alterations in H2O2-treated H9c2 cells could be ameliorated by GAS pretreatment. Moreover, we revealed that GAS pretreatment enhanced the nuclear translocation of Nrf2 under H2O2 treatment. Knockdown of Nrf2 expression abolished the protective effects of GAS on H2O2-treated H9c2 cells. Our results suggest that GAS may protect H9c2 cardiomycytes against oxidative injury via increasing the nuclear translocation of Nrf2, regulating mitochondrial dynamics, and maintaining the structure and functions of mitochondria.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P, et al. A review on central nervous system effects of gastrodin. Front Pharmacol. 2018;9:24.
Wu J, Wu B, Tang C, Zhao J. Analytical techniques and pharmacokinetics of gastrodia elata blume and its constituents. Molecules. 2017;22:E1137.
Feng L, Manavalan A, Mishra M, Sze SK, Hu JM, Heese K. Tianma modulates blood vessel tonicity. Open Biochem J. 2012;6:56–65.
Cheng Q, Yang W, Liu M. Research progress on pharmacological mechanism of Gastrodiae Rhizoma in Cardiovascular and metabolic diseases. Acad J Shanghai Univ Trad Chin Med. 2019;33:96–100.
Jiang J, Huang D, Li Y, Gan Z, Li H, Li X, et al. Heart protection by herb formula BanXia BaiZhu TianMa decoction in spontaneously hypertensive rats. Evid Based Complement Altern Med. 2019;2019:5612929.
Chen B, Wang Y, He Z, Wang D, Yan X, Xie P. Tianma Gouteng decoction for essential hypertension: protocol for a systematic review and meta-analysis. Evid Based Complement Altern Med. 2018;97:e9972.
Wei Y, Lin T, Jiawang D, Song L, Jian Y, Hui W, et al. Protective effect of gastrodin pretreatment on myocardial ischemia reperfusion injury. J Clin Cardiol. 2017;33:381–5.
Li Y, Wang X, Lou C. Gastrodin pretreatment impact on sarcoplasmic reticulum calcium transport ATPase (SERCA) and calcium phosphate (PLB) expression in rats with myocardial ischemia reperfusion. Med Sci Monit. 2016;22:3309–15.
Yao L, Ping Y, Hua H, Fei W, Junquan Y, Lin S, et al. Anti-apoptotic effect of gastrodin through inhibiting serum deprivation-induced autophagy in rat H9C2 cardiomyocytes. Chin J N Drugs. 2016;25:328–34.
Yang P, Han Y, Gui L, Sun J, Chen YL, Song R, et al. Gastrodin attenuation of the inflammatory response in H9c2 cardiomyocytes involves inhibition of NF-kappaB and MAPKs activation via the phosphatidylinositol 3-kinase signaling. Biochem Pharmacol. 2013;85:1124–33.
Yang M, Linn BS, Zhang Y, Ren J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2019;1865:2293–302.
Maneechote C, Palee S, Kerdphoo S, Jaiwongkam T, Chattipakorn SC, Chattipakorn N. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci. 2019;133:497–513.
Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. The role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidant. 2019;8:E454.
Ong SB, Hausenloy DJ. Mitochondrial dynamics as a therapeutic target for treating cardiac diseases. Handb Exp Pharmacol. 2017;240:251–79.
Yang Y, Zhao L, Ma J. Penehyclidine hydrochloride preconditioning provides cardiac protection in a rat model of myocardial ischemia/reperfusion injury via the mechanism of mitochondrial dynamics mechanism. Eur J Pharmacol. 2017;813:130–9.
Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62:341–60.
Wang W, Fernandez-Sanz C, Sheu SS. Regulation of mitochondrial bioenergetics by the non-canonical roles of mitochondrial dynamics proteins in the heart. Biochim Biophys Mol Basis Dis. 2018;1864:1991–2001.
Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am J Physiol Heart Circ Physiol. 2018;315:H1341–52.
Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–90.
Li X, Huang Q, Wang M, Yan X, Song X, Ma R, et al. Compound K inhibits autophagy-mediated apoptosis through activation of the PI3K-Akt signaling pathway thus protecting against ischemia/reperfusion injury. Cell Physiol Biochem. 2018;47:2589–601.
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12.
Cho SG, Xiao X, Wang S, Gao H, Rafikov R, Black S, et al. Bif-1 interacts with prohibitin-2 to regulate mitochondrial inner membrane during cell stress and apoptosis. J Am Soc Nephrol. 2019;30:1174–91.
Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19:630–41.
Chen Q, Chen X, Han C, Wang Y, Huang T, Du Y, et al. FGF-2 transcriptionally down-regulates the expression of BNIP3L via PI3K/Akt/FoxO3a signaling and inhibits necrosis and mitochondrial dysfunction induced by high concentrations of hydrogen peroxide in H9c2 cells. Cell Physiol Biochem. 2016;40:1678–91.
Li X, Zhu Q, Liu Y, Yang Z, Li B. Gastrodin protects myocardial cells against hypoxia/reoxygenation injury in neonatal rats by inhibiting cell autophagy through the activation of mTOR signals in PI3K-Akt pathway. J Pharm Pharmacol. 2018;70:259–67.
Li Y, Zhang Z. Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol. 2015;8:14099–109.
Jiang G, Hu Y, Liu L, Cai J, Peng C, Li Q. Gastrodin protects against MPP+-induced oxidative stress by up regulates heme oxygenase-1 expression through p38 MAPK/Nrf2 pathway in human dopaminergic cells. Neurochem Int. 2014;75:79–88.
Luo L, Kim SW, Lee HK, Kim ID, Lee H, Lee JK. Gastrodin exerts robust neuroprotection in the postischemic brain via its protective effect against Zn(2+)-toxicity and its anti-oxidative effects in astrocytes. Anim Cells Syst. 2018;22:429–37.
Liu S, Fang T, Yang L, Chen Z, Mu S, Fu Q. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway. Int J Mol Med. 2018;41:2059–69.
Huang Q, Shi J, Gao B, Zhang HY, Fan J, Li XJ, et al. Gastrodin: an ancient Chinese herbal medicine as a source for anti-osteoporosis agents via reducing reactive oxygen species. Bone. 2015;73:132–44.
Cao YP, Zheng M. Mitochondrial dynamics and inter-mitochondrial communication in the heart. Arch Biochem Biophys. 2019;663:214–9.
Chen Y, Liu Y, Dorn GW 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327–31.
Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400–14.
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22.
Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–9.
Karbowski M. Mitochondria on guard: role of mitochondrial fusion and fission in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:131–42.
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Devel Cell. 2001;1:515–25.
Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020;98:139–53.
Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5.
Li Y, Liu X. Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol. 2018;233:5589–97.
Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506.
Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–94.
Liu X, Weaver D, Shirihai O, Hajnoczky G. Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 2009;28:3074–89.
Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, et al. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol. 2014;204:919–29.
Jofuku A, Ishihara N, Mihara K. Analysis of functional domains of rat mitochondrial Fis1, the mitochondrial fission-stimulating protein. Biochem Biophys Res Commun. 2005;333:650–9.
Zhang Y, Chan DC. Structural basis for recruitment of mitochondrial fission complexes by Fis1. Proc Natl Acad Sci USA. 2007;104:18526–30.
Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–67.
Garcia I, Innis-Whitehouse W, Lopez A, Keniry M, Gilkerson R. Oxidative insults disrupt OPA1-mediated mitochondrial dynamics in cultured mammalian cells. Redox Rep. 2018;23:160–67.
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–47.
Covas G, Marinho HS, Cyrne L, Antunes F. Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. Methods Enzymol. 2013;528:157–71.
Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–62.
de Oliveira MR, Brasil FB, Furstenau CR. Evaluation of the mitochondria-related redox and bioenergetics effects of gastrodin in SH-SY5Y cells exposed to hydrogen peroxide. J Mol Neurosci. 2018;64:242–51.
Li Q, Niu C, Zhang X, Dong M. Gastrodin and isorhynchophylline synergistically inhibit MPP+-induced oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3beta pathways: involvement of Nrf2 nuclear translocation. ACS Chem Neurosci. 2018;9:482–93.
Zhao X, Zou Y, Xu H, Fan L, Guo H, Li X, et al. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012;1482:13–21.
Liu XC, Wu CZ, Hu XF, Wang TL, Jin XP, Ke SF, et al. Gastrodin attenuates neuronal apoptosis and neurological deficits after experimental intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020;29:104483.
de Oliveira MR, de Bittencourt Brasil F, Furstenau CR. Inhibition of the Nrf2/HO-1 axis suppresses the mitochondria-related protection promoted by gastrodin in human neuroblastoma cells exposed to paraquat. Mol Neurobiol. 2019;56:2174–84.
de Oliveira MR, Brasil FB, Furstenau CR. Nrf2 mediates the anti-apoptotic and anti-inflammatory effects induced by gastrodin in hydrogen peroxide-treated SH-SY5Y cells. J Mol Neurosci. 2019;69:115–22.
Liu S, Zhou L, Yang L, Mu S, Fang T, Fu Q. Gastrodin alleviates glucocorticoid induced osteoporosis in rats via activating the Nrf2 signaling pathways. Oncotarget. 2018;9:11528–40.
Qu LL, Yu B, Li Z, Jiang WX, Jiang JD, Kong WJ. Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway. Phytother Res. 2016;30:402–11.
Zhang H, Yuan B, Huang H, Qu S, Yang S, Zeng Z. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz J Med Biol Res. 2018;51:e7439.
Ong SB, Kalkhoran SB, Hernandez-Resendiz S, Samangouei P, Ong SG, Hausenloy DJ. Mitochondrial-shaping proteins in cardiac health and disease—the long and the short of it! Cardiovasc Drugs Ther. 2017;31:87–107.
Acknowledgements
This work was supported by the Chen-Kai-Xian Academician Workstation in Yunnan Province (No. 2017IC041), National Natural Science Foundation of China (Nos. 81873056 and 81872496), and the Open Project of the State Key Laboratory of Innovative Natural Medicine and Traditional Chinese Medicine Injections (No. QFSKL2018006).
Author information
Authors and Affiliations
Contributions
QQC conducted the experiments, analyzed the data, and drafted the paper. YWW participated in the experiments. WMY, MHT, YCW, and HYH helped to design the experiments. WDZ and XL supervised the research and revised the paper. All authors reviewed and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cheng, Qq., Wan, Yw., Yang, Wm. et al. Gastrodin protects H9c2 cardiomyocytes against oxidative injury by ameliorating imbalanced mitochondrial dynamics and mitochondrial dysfunction. Acta Pharmacol Sin 41, 1314–1327 (2020). https://doi.org/10.1038/s41401-020-0382-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0382-x
Keywords
This article is cited by
-
Bridging tradition and modernity: mitochondrial dynamics as a Traditional Chinese Medicine therapeutic target in cardiovascular disease
Chinese Medicine (2026)
-
MiR221/222 in the conditioned medium of adipose-derived stem cells attenuates particulate matter and high-fat diet-induced cardiac apoptosis
Stem Cell Research & Therapy (2025)
-
Suppressing mitochondrial inner membrane protein (IMMT) inhibits the proliferation of breast cancer cells through mitochondrial remodeling and metabolic regulation
Scientific Reports (2024)
-
Mitochondria-Associated Organelle Crosstalk in Myocardial Ischemia/Reperfusion Injury
Journal of Cardiovascular Translational Research (2024)
-
Gastrodin: a comprehensive pharmacological review
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)