Abstract
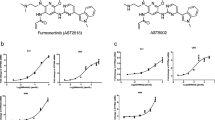
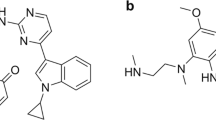
Alflutinib (AST2818) is a third-generation epidermal growth factor receptor (EGFR) inhibitor that inhibits both EGFR-sensitive mutations and T790M mutations. Previous study has shown that after multiple dosages, alflutinib exhibits nonlinear pharmacokinetics and displays a time- and dose-dependent increase in the apparent clearance, probably due to its self-induction of cytochrome P450 (CYP) enzyme. In this study, we investigated the CYP isozymes involved in the metabolism of alflutinib and evaluated the enzyme inhibition and induction potential of alflutinib and its metabolites. The data showed that alflutinib in human liver microsomes (HLMs) was metabolized mainly by CYP3A4, which could catalyze the formation of AST5902. Alflutinib did not inhibit CYP isozymes in HLMs but could induce CYP3A4 in human hepatocytes. Rifampin is a known strong CYP3A4 inducer and is recommended by the FDA as a positive control in the CYP3A4 induction assay. We found that the induction potential of alflutinib was comparable to that of rifampin. The Emax of CYP3A4 induction by alflutinib in three lots of human hepatocytes were 9.24-, 11.2-, and 10.4-fold, while the fold-induction of rifampin (10 μM) were 7.22-, 19.4- and 9.46-fold, respectively. The EC50 of alflutinib-induced CYP3A4 mRNA expression was 0.25 μM, which was similar to that of rifampin. In addition, AST5902 exhibited much weak CYP3A4 induction potential compared to alflutinib. Given the plasma exposure of alflutinib and AST5902, both are likely to affect the pharmacokinetics of CYP3A4 substrates. Considering that alflutinib is a CYP3A4 substrate and a potent CYP3A4 inducer, drug–drug interactions are expected during alflutinib treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019;39:22.
Sun S, Schiller JH, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740–50.
Alvarez M, Roman E, Santos ES. New targets for non-small-cell lung cancer therapy. Expert Rev Anticanc. 2007;7:1423–37.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk Factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–54.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–81.
Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29:2866–74.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
Paz-Ares L, Tan EH, O’Byrne K, Zhang L, Hirsh V, Boyer M, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28:270–7.
Kim Y, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Efficacy and safety of afatinib for EGFR-mutant non-small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat. 2019;51:502–9.
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66.
Ramalingam SS, O’Byrne K, Boyer M, Mok T, Janne PA, Zhang H, et al. Dacomitinib versus erlotinib in patients with EGFR-mutated advanced nonsmall-cell lung cancer (NSCLC): pooled subset analyses from two randomized trials. Ann Oncol. 2016;27:423–9.
Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. Plos Med. 2005;2:225–35.
Han W, Du Y. Recent development of the second and third generation irreversible epidermal growth factor receptor inhibitors. Chem Biodivers. 2017;14:e1600372.
Cheng H, Nair SK, Murray BW. Recent progress on third generation covalent EGFR inhibitors. Bioorg Med Chem Lett. 2016;26:1861–8.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Kim ES. Olmutinib: first global approval. Drugs. 2016;76:1153–7.
Shi YK, Zhang SC, Hu XS, Feng JF, Ma ZY, Zhou JY, et al. Safety, clinical activity and pharmacokinetics of alflutinib (AST2818) in advanced NSCLC patients with EGFR T790M mutation. J Thorac Oncol. 2020; in press.
Liu XY, Li W, Zhang YF, Jiang Y, Zhao QY, Zhong DF. Simultaneous determination of alflutinib and its active metabolite in human plasma using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2019;176:112735.
Rodrigues AD. Integrated cytochrome P450 reaction phenotyping. Biochem Pharmacol. 1999;57:465–80.
Fahmi OA, Ripp SL. Evaluation of models for predicting drug–drug interactions due to induction. Expert Opin Drug Met. 2010;6:1399–416.
Xu Y, Zhou Y, Hayashi M, Shou M, Skiles GL. Simulation of clinical drug-drug interactions from hepatocyte CYP3A4 induction data and its potential utility in trial designs. Drug Metab Dispos. 2011;39:1139–48.
Shou M, Hayashi M, Pan Y, Xu Y, Morrissey K, Xu L, et al. Modeling, prediction, and in vitro in vivo correlation of CYP3A4 induction. Drug Metab Dispos. 2008;36:2355–70.
Dixit V, Moore A, Tsao H, Hariparsad N. Application of micropatterned cocultured hepatocytes to evaluate the inductive potential and degradation rate of major xenobiotic metabolizing enzymes. Drug Metab Dispos. 2016;44:250–61.
Garg V, Chandorkar G, Yang Y, Adda N, McNair L, Alves K, et al. The effect of CYP3A inhibitors and inducers on the pharmacokinetics of telaprevir in healthy volunteers. Br J Clin Pharmacol. 2013;75:431–9.
Dickinson PA, Cantarini MV, Collier J, Frewer P, Martin S, Pickup K, et al. Metabolic disposition of osimertinib in rats, dogs, and humans: insights into a drug designed to bind covalently to a cysteine residue of epidermal growth factor receptor. Drug Metab Dispos. 2016;44:1201–12.
Vishwanathan K, Dickinson PA, So K, Thomas K, Chen YM, De Castro Carpeno J, et al. The effect of itraconazole and rifampicin on the pharmacokinetics of osimertinib. Br J Clin Pharmacol. 2018;84:1156–69.
Tolson AH, Wang H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev. 2010;62:1238–49.
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences [XDA12050306] and the National Natural Science Foundation of China [81521005, 81903701].
Author information
Authors and Affiliations
Contributions
XYL, DFZ, YJ, and QYZ participated in the research design; XYL, ZTG, ZDC, and JLZ conducted the experiments; XYL and YFZ performed data analysis; XYL, DFZ, and XXD wrote or contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Xy., Guo, Zt., Chen, Zd. et al. Alflutinib (AST2818), primarily metabolized by CYP3A4, is a potent CYP3A4 inducer. Acta Pharmacol Sin 41, 1366–1376 (2020). https://doi.org/10.1038/s41401-020-0389-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0389-3
Keywords
This article is cited by
-
Metabolic disposition of the EGFR covalent inhibitor furmonertinib in humans
Acta Pharmacologica Sinica (2022)
-
Furmonertinib (Alflutinib, AST2818) is a potential positive control drug comparable to rifampin for evaluation of CYP3A4 induction in sandwich-cultured primary human hepatocytes
Acta Pharmacologica Sinica (2022)
-
Effect of autoinduction and food on the pharmacokinetics of furmonertinib and its active metabolite characterized by a population pharmacokinetic model
Acta Pharmacologica Sinica (2022)
-
Genetic landscape of 125 pharmacogenes in Chinese from the Chinese Millionome Database
Scientific Reports (2021)
-
Effects of rifampicin on the pharmacokinetics of alflutinib, a selective third-generation EGFR kinase inhibitor, and its metabolite AST5902 in healthy volunteers
Investigational New Drugs (2021)