Abstract
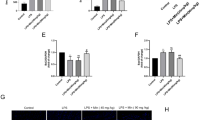
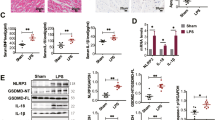
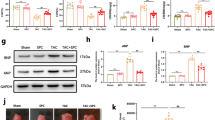
In patients with sepsis, lipopolysaccharide (LPS) from the outer membrane of gram-negative bacteria triggers cardiac dysfunction and heart failure, but target therapy for septic cardiomyopathy remains unavailable. In this study we evaluated the beneficial effects of cardamonin (CAR), a flavone existing in Alpinia plant, on endotoxemia-induced cardiac dysfunction and the underlying mechanisms with focus on oxidative stress and apoptosis. Adult mice were exposed to LPS (4 mg/kg, i.p. for 6 h) prior to functional or biochemical assessments. CAR (20 mg/kg, p.o.) was administered to mice immediately prior to LPS challenge. We found that LPS challenge compromised cardiac contractile function, evidenced by compromised fractional shortening, peak shortening, maximal velocity of shortening/relengthening, enlarged LV end systolic diameter and prolonged relengthening in echocardiography, and induced apoptosis, overt oxidative stress (O2− production and reduced antioxidant defense) associated with inflammation, phosphorylation of NF-κB and cytosolic translocation of transcriptional factor Nrf2. These deteriorative effects were greatly attenuated or mitigated by CAR administration. However, H&E and Masson’s trichrome staining analysis revealed that neither LPS challenge nor CAR administration significantly affected cardiomyocyte cross-sectional area and interstitial fibrosis. Mouse cardiomyocytes were treated with LPS (4 µg/mL) for 6 h in the absence or presence of CAR (10 μM) in vitro. We found that addition of CAR suppressed LPS-induced defect in cardiomyocyte shortening, which was nullified by the Nrf2 inhibitor ML-385 or the NF-κB activator prostratin. Taken together, our results suggest that CAR administration protects against LPS-induced cardiac contractile abnormality, oxidative stress, apoptosis, and inflammation through Nrf2- and NF-κB-dependent mechanism.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arfaras-Melainis A, Polyzogopoulou E, Triposkiadis F, Xanthopoulos A, Ikonomidis I, Mebazaa A, et al. Heart failure and sepsis: practical recommendations for the optimal management. Heart Fail Rev. 2020;25:183–94.
Ceylan-Isik AF, Zhao P, Zhang B, Xiao X, Su G, Ren J. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol. 2010;48:367–78.
Turdi S, Han X, Huff AF, Roe ND, Hu N, Gao F, et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced myocardial contractile dysfunction: role of autophagy. Free Radic Biol Med. 2012;53:1327–38.
Zhang Y, Xu X, Ceylan-Isik AF, Dong M, Pei Z, Li Y, et al. Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6. J Mol Cell Cardiol. 2014;74:76–87.
Coverstone ED, Bach RG, Chen L, Bierut LJ, Li AY, Lenzini PA, et al. A novel genetic marker of decreased inflammation and improved survival after acute myocardial infarction. Basic Res Cardiol. 2018;113:38.
Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, et al. Brain natriuretic peptide: a marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–5.
Ren J, Wu S. A burning issue: do sepsis and systemic inflammatory response syndrome (SIRS) directly contribute to cardiac dysfunction? Front Biosci. 2006;11:15–22.
Stanzani G, Duchen MR, Singer M. The role of mitochondria in sepsis-induced cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:759–73.
Tan Y, Chen S, Zhong J, Ren J, Dong M. Mitochondrial injury and targeted intervention in septic cardiomyopathy. Curr Pharmacol Des. 2019;25:2060–70.
Durand A, Duburcq T, Dekeyser T, Neviere R, Howsam M, Favory R, et al. Involvement of mitochondrial disorders in septic cardiomyopathy. Oxid Med Cell Longev. 2017;2017:4076348.
Soriano FG, Lorigados CB, Pacher P, Szabo C. Effects of a potent peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis. Shock. 2011;35:560–6.
Torres-Duenas D, Celes MR, Freitas A, Alves-Filho JC, Spiller F, Dal-Secco D, et al. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br J Pharmacol. 2007;152:341–52.
Pang J, Peng H, Wang S, Xu X, Xu F, Wang Q, et al. Mitochondrial ALDH2 protects against lipopolysaccharide-induced myocardial contractile dysfunction by suppression of ER stress and autophagy. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1627–41.
Mahmoud AM, Hernandez Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138.
Fan XJ, Ren J. Compensation: a contemporary regulatory machinery in cardiovascular diseases? Cardiovasc Toxicol. 2012;12:275–84.
Fan XJ, Yu H, Ren J. Homeostasis and compensatory homeostasis: bridging western medicine and traditional chinese medicine. Curr Cardiol Rev. 2011;7:43–6.
Li YY, Huang SS, Lee MM, Deng JS, Huang GJ. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-kappaB and MAPK signaling pathway in the carrageenan-induced paw edema. Int Immunopharmacol. 2015;25:332–9.
Peng S, Hou Y, Yao J, Fang J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017;8:997–1007.
Jantan I, Raweh SM, Sirat HM, Jamil S, Mohd Yasin YH, Jalil J, et al. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine. 2008;15:306–9.
Wei Z, Yang J, Xia YF, Huang WZ, Wang ZT, Dai Y. Cardamonin protects septic mice from acute lung injury by preventing endothelial barrier dysfunction. J Biochem Mol Toxicol. 2012;26:282–90.
Wang Z, Xu G, Gao Y, Zhan X, Qin N, Fu S, et al. Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B. 2019;9:734–44.
Li W, Wu X, Li M, Wang Z, Li B, Qu X, et al. Cardamonin alleviates pressure overload-induced cardiac remodeling and dysfunction through inhibition of oxidative stress. J Cardiovasc Pharmacol. 2016;68:441–51.
Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells. Mol Immunol. 2007;44:673–9.
Lee M-Y, Seo C-S, Lee J-A, Shin I-S, Kim S-J, Ha H, et al. Alpinia katsumadai HAYATA seed extract inhibit LPS-induced inflammation by induction of heme oxygenase-1 in RAW264.7. Cells. 2012;35:746–57.
Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol. 2016;93:18–31.
Sun Y, Cai Y, Zang QS. Cardiac autophagy in sepsis. Cells. 2019;8:141.
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, et al. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247–62.
Dong M, Hu N, Hua Y, Xu X, Kandadi MR, Guo R, et al. Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3beta-dependent inhibition of apoptosis and ER stress. Biochim Biophys Acta. 2013;1832:848–63.
Koentges C, Cimolai MC, Pfeil K, Wolf D, Marchini T, Tarkhnishvili A, et al. Impaired SIRT3 activity mediates cardiac dysfunction in endotoxemia by calpain-dependent disruption of ATP synthesis. J Mol Cell Cardiol. 2019;133:138–47.
Wang S, Zhu X, Xiong L, Ren J. Ablation of Akt2 prevents paraquat-induced myocardial mitochondrial injury and contractile dysfunction: role of Nrf2. Toxicol Lett. 2017;269:1–14.
Ceylan AF, Wang S, Kandadi MR, Chen J, Hua Y, Pei Z, et al. Cardiomyocyte-specific knockout of endothelin receptor a attenuates obesity cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3339–52.
Li SY, Gilbert SA, Li Q, Ren J. Aldehyde dehydrogenase-2 (ALDH2) ameliorates chronic alcohol ingestion-induced myocardial insulin resistance and endoplasmic reticulum stress. J Mol Cell Cardiol. 2009;47:247–55.
Li D, Qi J, Wang J, Pan Y, Li J, Xia X, et al. Protective effect of dihydroartemisinin in inhibiting senescence of myeloid-derived suppressor cells from lupus mice via Nrf2/HO-1 pathway. Free Radic Biol Med. 2019;143:260–74.
Chen D, Wang H, Aweya JJ, Chen Y, Chen M, Wu X, et al. HMBA enhances prostratin-induced activation of latent HIV-1 via suppressing the expression of negative feedback regulator A20/TNFAIP3 in NF-kappaB signaling. Biomed Res Int. 2016;2016:5173205.
Wang Z, Zhang Y, Guo J, Jin K, Li J, Guo X, et al. Inhibition of protein kinase C betaII isoform rescues glucose toxicity-induced cardiomyocyte contractile dysfunction: role of mitochondria. Life Sci. 2013;93:116–24.
Zhang Y, Xia Z, La Cour KH, Ren J. Activation of Akt rescues endoplasmic reticulum stress-impaired murine cardiac contractile function via glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeation pore opening. Antioxid Redox Signal. 2011;15:2407–24.
Hasna J, Hague F, Rodat-Despoix L, Geerts D, Leroy C, Tulasne D, et al. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: the p53 connection. Cell Death Differ. 2018;25:693–707.
Wei Y, Chang Y, Zeng H, Liu G, He C, Shi H. RAV transcription factors are essential for disease resistance against cassava bacterial blight via activation of melatonin biosynthesis genes. J Pineal Res. 2018;64:10.
Ren J, Ren BH, Sharma AC. Sepsis-induced depressed contractile function of isolated ventricular myocytes is due to altered calcium transient properties. Shock. 2002;18:285–8.
Lew WY, Bayna E, Dalle Molle E, Contu R, Condorelli G, Tang T. Myocardial fibrosis induced by exposure to subclinical lipopolysaccharide is associated with decreased miR-29c and enhanced NOX2 expression in mice. PLoS ONE. 2014;9:e107556.
Lew WY, Bayna E, Molle ED, Dalton ND, Lai NC, Bhargava V, et al. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS ONE. 2013;8:e61057.
Gasteiger G, D’Osualdo A, Schubert DA, Weber A, Bruscia EM, Hartl D. Cellular innate immunity: an old game with new players. J Innate Immun. 2017;9:111–25.
Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY. Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J Immunol. 2015;195:4999–5010.
Vomund S, Schafer A, Parnham MJ, Brune B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18:E2772.
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun. 2016;7:11624.
Li J, Zhang C, Xing Y, Janicki JS, Yamamoto M, Wang XL, et al. Up-regulation of p27(kip1) contributes to Nrf2-mediated protection against angiotensin II-induced cardiac hypertrophy. Cardiovascular Res. 2011;90:315–24.
Biswal S, Thimmulappa RK, Harvey CJ. Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proc Am Thorac Soc. 2012;9:47–51.
Walsh J, Jenkins RE, Wong M, Olayanju A, Powell H, Copple I, et al. Identification and quantification of the basal and inducible Nrf2-dependent proteomes in mouse liver: biochemical, pharmacological and toxicological implications. J Proteom. 2014;108:171–87.
Singh A, Venkannagari S, Oh KH, Zhang YQ, Rohde JM, Liu L, et al. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-Deficient NSCLC tumors. ACS Chem Biol. 2016;11:3214–25.
Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, et al. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev. 2018;70:348–83.
Lu MC, Ji JA, Jiang ZY, You QD. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev. 2016;36:924–63.
de Freitas Silva M, Pruccoli L, Morroni F, Sita G, Seghetti F, Viegas C, et al. The Keap1/Nrf2-ARE pathway as a pharmacological target for chalcones. Molecules. 2018;23:E1803.
Abed DA, Goldstein M, Albanyan H, Jin H, Hu L. Discovery of direct inhibitors of Keap1-Nrf2 protein–protein interaction as potential therapeutic and preventive agents. Acta Pharm Sin B. 2015;5:285–99.
Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116.
Dinkova-Kostova AT, Liby K, Stephenson KK, Holtzclaw WD, Gao X, Suh N, et al. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci USA. 2005;102:4584–9.
Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64:972–1003.
Zagorski JW, Turley AE, Freeborn RA, VanDenBerg KR, Dover HE, Kardell BR, et al. Differential effects of the Nrf2 activators tBHQ and CDDO-Im on the early events of T cell activation. Biochem Pharmacol. 2018;147:67–76.
Qi W, Boliang W, Xiaoxi T, Guoqiang F, Jianbo X, Gang W. Cardamonin protects against doxorubicin-induced cardiotoxicity in mice by restraining oxidative stress and inflammation associated with Nrf2 signaling. Biomed Pharmacother. 2020;122:109547.
De Spirt S, Eckers A, Wehrend C, Micoogullari M, Sies H, Stahl W, et al. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic Biol Med. 2016;91:164–71.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81671938, 81571895).
Author information
Authors and Affiliations
Contributions
YT, HHW, MMS, WJZ, MLD, and WG performed the experimental study; JR, MLD, and HP conceived and designed the study and drafted, edited and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Tan, Y., Wan, Hh., Sun, Mm. et al. Cardamonin protects against lipopolysaccharide-induced myocardial contractile dysfunction in mice through Nrf2-regulated mechanism. Acta Pharmacol Sin 42, 404–413 (2021). https://doi.org/10.1038/s41401-020-0397-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0397-3
Keywords
This article is cited by
-
Comprehensive insights into sepsis-induced cardiac dysfunction through proteomics and metabolomics
BMC Research Notes (2026)
-
The role of programmed cell death in organ dysfunction induced by opportunistic pathogens
Critical Care (2025)
-
Nrf2 mediated signaling axis in sepsis-induced cardiomyopathy: potential Pharmacological receptor
Inflammation Research (2025)
-
Yoda1/Piezo1 Alleviates Lipopolysaccharide-Induced Cardiac Injury via AMPK-Mediated Mitophagy
Cardiovascular Toxicology (2025)
-
Circulating Biomarkers to Predict Post-Operative Cognitive Decline in Patients Undergoing Coronary Artery Bypass Grafting
Cellular and Molecular Neurobiology (2025)