Abstract
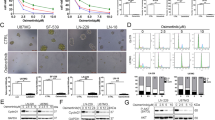
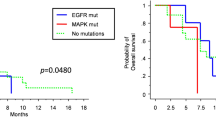
Glioblastoma (GBM) patients have extremely poor prognoses, and currently no effective treatment available including surgery, radiation, and chemotherapy. MAPK-interacting kinases (MNK1/2) as the downstream of the MAPK-signaling pathway regulate protein synthesis in normal and tumor cells. Research has shown that targeting MNKs may be an effective strategy to treat GBM. In this study we investigated the antitumor activity of osimertinib, an FDA-approved epidermal growth factor receptor (EGFR) inhibitor, against patient-derived primary GBM cells. Using high-throughput screening approach, we screened the entire panel of FDA-approved drugs against primary cancer cells derived from glioblastoma patients, found that osimertinib (3 μM) suppressed the proliferation of a subset (10/22) of EGFR-negative GBM cells (>50% growth inhibition). We detected the gene expression difference between osimertinib-sensitive and -resistant cells, found that osimertinib-sensitive GBM cells displayed activated MAPK-signaling pathway. We further showed that osimertinib potently inhibited the MNK kinase activities with IC50 values of 324 nM and 48.6 nM, respectively, against MNK1 and MNK2 kinases; osimertinib (0.3–3 μM) dose-dependently suppressed the phosphorylation of eukaryotic translation initiation factor 4E (eIF4E). In GBM patient-derived xenografts mice, oral administration of osimertinib (40 mg· kg−1 ·d−1, for 18 days) significantly suppressed the tumor growth (TGI = 74.5%) and inhibited eIF4E phosphorylation in tumor cells. Given the fact that osimertinib could cross the blood–brain barrier and its toxicity was well tolerated in patients, our results suggest that osimertinib could be a new and effective drug candidate for the EGFR-negative GBM patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nizamutdinov D, Stock EM, Dandashi JA, Vasquez EA, Mao Y, Dayawansa S, et al. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018;109:e67–e74.
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18:3–9.
de Robles P, Fiest KM, Frolkis AD, Pringsheim T, Atta C, St Germaine-Smith C, et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro Oncol. 2015;17:776–83.
Bell JB, Eckerdt FD, Alley K, Magnusson LP, Hussain H, Bi Y, et al. MNK inhibition disrupts mesenchymal glioma stem cells and prolongs survival in a mouse model of glioblastoma. Mol Cancer Res. 2016;14:984–93.
Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–33.
Proud CG. Mnks, eIF4E phosphorylation and cancer. Biochim Biophys Acta. 2015;1849:766–73.
Graff JR, Konicek BW, Carter JH, Marcusson EG. Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res. 2008;68:631–4.
Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–6.
Hou J, Lam F, Proud C, Wang S. Targeting mnks for cancer therapy. Oncotarget. 2012;3:118–31.
Diab S, Kumarasiri M, Yu M, Teo T, Proud C, Milne R, et al. MAP kinase-interacting kinases-emerging targets against cancer. Chem Biol. 2014;21:441–52.
Truitt ML, Conn CS, Shi Z, Pang X, Tokuyasu T, Coady AM, et al. Differential requirements for eIF4E dose in normal development and cancer. Cell. 2015;162:59–71.
Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6:275–7.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–61.
Lee SY. Temozolomide resistance in glioblastoma multiforme. Gene Dis. 2016;3:198–210.
Colclough N, Ballard PG, Barton P, Chen K, Cross DAE, Finlay MRV, et al. Preclinical comparison of the blood brain barrier (BBB) permeability of osimertinib (AZD9291) with other irreversible next generation EGFR TKIs. Eur J Cancer. 2016;69:S28.
Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–40.
Liu X, Chen X, Shi L, Shan Q, Cao Q, Yue C, et al. The third-generation EGFR inhibitor AZD9291 overcomes primary resistance by continuously blocking ERK signaling in glioblastoma. J Exp Clin Cancer Res. 2019;38:219.
Grzmil M, Seebacher J, Hess D, Behe M, Schibli R, Moncayo G, et al. Inhibition of MNK pathways enhances cancer cell response to chemotherapy with temozolomide and targeted radionuclide therapy. Cell Signal. 2016;28:1412–21.
Grzmil M, Morin P Jr, Lino MM, Merlo A, Frank S, Wang Y, et al. MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res. 2011;71:2392–402.
Bell JB, Eckerdt FD, Alley K, Magnusson LP, Hussain H, Bi Y, et al. MNK inhibition disrupts mesenchymal glioma stem cells and prolongs survival in a mouse model of glioblastoma. Mol Cancer Res. 2016;14:984–93.
Finlay MR, Anderton M, Ashton S, Ballard P, Bethel PA, Box MR, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57:8249–67.
Gilani A, Donson A, Davies KD, Whiteway SL, Lake J, DeSisto J, et al. Targetable molecular alterations in congenital glioblastoma. J Neurooncol. 2020;146:247–52.
Chiba R, Akiya M, Hashimura M, Oguri Y, Inukai M, Hara A, et al. ALK signaling cascade confers multiple advantages to glioblastoma cells through neovascularization and cell proliferation. PLoS ONE. 2017;12:e0183516.
Junca A, Villalva C, Tachon G, Rivet P, Cortes U, Guilloteau K, et al. Crizotinib targets in glioblastoma stem cells. Cancer Med. 2017;6:2625–34.
Jimenez-Pascual A, Siebzehnrubl FA. Fibroblast growth factor receptor functions in glioblastoma. Cells. 2019;8:E715.
Natarajan M, Hecker TP, Gladson CL. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 2003;9:126–33.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grants 81773777, 81673469, 81703559, and 81172407), the Natural Science Foundation of Anhui Province (grants 1808085MH274 and 1908085MH259), the China Post-doctoral Science Foundation (grants 2018T110634, 2018M630720, and 2019M652057), the Post-doctoral Science Foundation of Anhui Province (grant 2018B279), Science and Technology Project grants from Anhui Province (grants 1508085QHl84, 1606c08235, and 1604a0802069), the Fundamental Research Fund for Central Universities (WK 9110000032), the Frontier Science Key Research Program of the Chinese Academy of Sciences (grant QYZDB-SSW-SLH037), the CASHIPS Director’s Fund (grant BJPY2019A03), the Key Program of the 13th Five-Year Plan of CASHIPS (grant KP-2017-26), and the Presidential Foundation of CASHIPS (grant YZJJ2018QN17). A portion of this work was supported by the High Magnetic Field Laboratory of Anhui Province.
Author information
Authors and Affiliations
Contributions
QSL, CSN, and WCW designed the project and wrote the manuscript. CC, CDC, HW, LW, ZWW, and ZRJ performed the experiments and collected the data. CC, HW, ALW, and CH drafted the manuscript. YFD, WXN, and SQ analyzed the data. ZPQ and JL revised the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, C., Cheng, Cd., Wu, H. et al. Osimertinib successfully combats EGFR-negative glioblastoma cells by inhibiting the MAPK pathway. Acta Pharmacol Sin 42, 108–114 (2021). https://doi.org/10.1038/s41401-020-0418-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0418-2
Keywords
This article is cited by
-
Application of EGFR-TKIs in brain tumors, a breakthrough in future?
Journal of Translational Medicine (2025)
-
Overcoming temozolomide resistance in glioma: recent advances and mechanistic insights
Acta Neuropathologica Communications (2025)
-
It’s all downstream from here: RTK/Raf/MEK/ERK pathway resistance mechanisms in glioblastoma
Journal of Neuro-Oncology (2025)
-
DDX3X dynamics, glioblastoma's genetic landscape, therapeutic advances, and autophagic interplay
Medical Oncology (2024)
-
Signaling pathways in brain tumors and therapeutic interventions
Signal Transduction and Targeted Therapy (2023)