Abstract
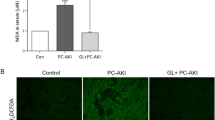
Mitsugumin 53 (MG53) is a tripartite motif family protein that has been reported to attenuate injury via membrane repair in different organs. Contrast-induced acute kidney injury (CI-AKI) is a common complication caused by the administration of iodinated contrast media (CM). While the cytotoxicity induced by CM leading to tubular cell death may be initiated by cell membrane damage, we wondered whether MG53 alleviates CI-AKI. This study was designed to investigate the effect of MG53 on CI-AKI and the underlying mechanism. A rat model of CI-AKI was established, and CI-AKI induced the translocation of MG53 from serum to injury sites on the renal proximal tubular (RPT) epithelia, as illustrated by immunoblot analysis and immunohistochemical staining. Moreover, pretreatment of rats with recombinant human MG53 protein (rhMG53, 2 mg/mL) alleviated iopromide-induced injury in the kidney, which was determined by measuring serum creatinine, blood urea nitrogen and renal histological changes. In vitro studies demonstrated that exposure of RPT cells to iopromide (20, 40, and 80 mg/mL) caused cell membrane injury and cell death, which were attenuated by rhMG53 (10 and 50 μg/mL). Mechanistically, MG53 translocated to the injury site on RPT cells and bound to phosphatidylserine to protect RPT cells from iopromide-induced injury. In conclusion, MG53 protects against CI-AKI through cell membrane repair and reducing cell apoptosis; therefore, rhMG53 might be a potential effective means to treat or prevent CI-AKI.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Calvin AD, Misra S, Pflueger A. Contrast-induced acute kidney injury and diabetic nephropathy. Nat Rev Nephrol. 2010;6:679–88.
Davidson CJ, Hlatky M, Morris KG, Pieper K, Skelton TN, Schwab SJ, et al. Cardiovascular and renal toxicity of a nonionic radiographic contrast agent after cardiac catheterization. A prospective trial. Ann Intern Med. 1989;110:119–24.
McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–28.
Fähling M, Seeliger E, Patzak A, Persson PB. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol. 2017;13:169–80.
Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177–82.
Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–71.
Rancic ZS. Commentary on 'Contrast Induced Nephropathy and Long-term Renal Decline After Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease'. Eur J Vasc Endovasc Surg. 2016;51:394.
Tepel M, Aspelin P, Lameire N. Contrast-induced nephropathy: a clinical and evidence-based approach. Circulation. 2006;113:1799–806.
Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–15.
Silvain J, Collet JP, Montalescot G. Contrast-induced nephropathy: the sin of primary percutaneous coronary intervention? Eur Heart J. 2014;35:1504–6.
Romano G, Briguori C, Quintavalle C, Zanca C, Rivera NV, Colombo A, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008;29:2569–76.
Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, Heretis I, Wilks MF, Spandidos DA, et al. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017;180:99–112.
Mathew R, Haque K, Woothipoom W. Acute renal failure induced by contrast medium: steps towards prevention. BMJ. 2006;333:539–40.
ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation. 2011;124:1250–9.
Thiele H, Hildebrand L, Schirdewahn C, Eitel I, Adams V, Fuernau G, et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55:2201–9.
Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82.
Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–94.
Myrvang H. Acute kidney injury: Acetylcysteine does not prevent contrast-induced acute kidney injury. Nat Rev Nephrol. 2011;7:605.
Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21.
Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. ESCRT machinery is required for plasma membrane repair. Science. 2014;343:1247136.
Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol. 2011;38:292–9.
Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, et al. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol. 2009;11:56–64.
Yi JS, Park JS, Ham YM, Nguyen N, Lee NR, Hong J, et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun. 2013;4:2354.
Tan T, Ko YG, Ma J. Dual function of MG53 in membrane repair and insulin signaling. BMB Rep. 2016;49:414–23.
Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 2012;4:139ra85.
Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R, et al. Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat Commun. 2014;5:4387.
Liu J, Zhu H, Zheng Y, Xu Z, Li L, Tan T, et al. Cardioprotection of recombinant human MG53 protein in a porcine model of ischemia and reperfusion injury. J Mol Cell Cardiol. 2015;80:10–9.
Chandler HL, Tan T, Yang C, Gemensky-Metzler AJ, Wehrman RF, Jiang Q, et al. MG53 promotes corneal wound healing and mitigates fibrotic remodeling in rodents. Commun Biol. 2019;2:71.
Pu D, Li H, Lin P, Tan T, Wang Z, Chen K, et al. MG53-mediated cell membrane repair protects against acute kidney injury. Sci Transl Med. 2015;7:279ra36.
Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–75.
Yokomaku Y, Sugimoto T, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, et al. Asialoerythropoietin prevents contrast-induced nephropathy. J Am Soc Nephrol. 2008;19:321–8.
Chen YH, Fu YC, Wu MJ. Does Resveratrol Play a Role in Decreasing the Inflammation Associated with Contrast Induced Nephropathy in Rat Model? J Clin Med. 2019;8:147.
Wang Z, Liu Y, Han Y, Guan W, Kou X, Fu J, et al. Protective effects of aliskiren on ischemia–reperfusion-induced renal injury in rats. Eur J Pharmacol. 2013;718:160–6.
Li H, Duann P, Lin PH, Zhao L, Fan Z, Tan T, et al. Modulation of wound healing and scar formation by MG53 protein-mediated cell membrane repair. J Biol Chem. 2015;290:24592–603.
Yao Y, Zhang B, Zhu H, Li H, Han Y, Chen K, et al. MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget. 2016;7:22474–85.
Weisleder N, Lin P, Zhao X, Orange M, Zhu H, Ma J. Visualization of MG53-mediated cell membrane repair using in vivo and in vitro systems. J Vis Exp. 2011;52:2717.
Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol. 2009;2:225–6.
Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem. 2009;284:15894–902.
Zhang Y, Lv F, Jin L, Peng W, Song R, Ma J, et al. MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res. 2011;91:108–15.
Wu Y, Huang J, Liu D, Tan J, Peng Y, Yang J, et al. Mitsugumin 53 protects the kidney from severe burn injury in mice. Burns Trauma. 2013;1:128–33.
Michael A, Faga T, Pisani A, Riccio E, Bramanti P, Sabbatini M, et al. Molecular mechanisms of renal cellular nephrotoxicity due to radiocontrast media. Biomed Res Int. 2014;2014:249810.
Liu ZZ, Schmerbach K, Lu Y, Perlewitz A, Nikitina T, Cantow K, et al. Iodinated contrast media cause direct tubular cell damage, leading to oxidative stress, low nitric oxide, and impairment of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2014;306:F864–72.
Zager RA, Johnson ACM, Hanson SY. Radiographic contrast media-induced tubular injury: Evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;64:128–39.
Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, et al. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem. 2011;286:12820–4.
Acknowledgements
These studies were supported in part by grants to CZ from the National Key R&D Program of China (2018YFC1312700), National Natural Science Foundation of China (31730043, 81600585), Program of Innovative Research Team by National Natural Science Foundation of China (81721001), Chongqing Technology Innovation and Application Demonstration Project (cstc2018jscx-mszdX0024), and Clinical Medical Research Personnel Training Program of the Army Medical University (2018XLC10I2). Research conducted in the laboratories of JJM and BHR was supported by the US National Institutes of Health (R01-DK106394).
Author information
Authors and Affiliations
Contributions
CYZ, YH, and CL designed the experiments. CL, YHH, YBW, YZ, XQZ, DFH, HMR, YKL, PHL, and HCL performed the experiments and analyzed the data. CL, YHH, and HYW prepared the original manuscript. TT, BHR, JJM, and CYZ revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
JJM and TT have an equity interest in TRIM-edicine, Inc., which develops rhMG53 for the treatment of human diseases. Patents on the use of MG53 are held by Rutgers University—Robert Wood Johnson Medical School.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, C., Hu, Yh., Han, Y. et al. MG53 protects against contrast-induced acute kidney injury by reducing cell membrane damage and apoptosis. Acta Pharmacol Sin 41, 1457–1464 (2020). https://doi.org/10.1038/s41401-020-0420-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0420-8
Keywords
This article is cited by
-
Machine learning-guided Nano-QSAR modeling predicts HepaRG cell membrane toxicity of engineered nanoparticles with mechanistic insights
Cell Biology and Toxicology (2026)
-
Multipronged regulation of autophagy and apoptosis: emerging role of TRIM proteins
Cellular & Molecular Biology Letters (2024)
-
Structural basis for TRIM72 oligomerization during membrane damage repair
Nature Communications (2023)
-
Increased AT1 receptor expression mediates vasoconstriction leading to hypertension in Snx1−/− mice
Hypertension Research (2021)