Abstract
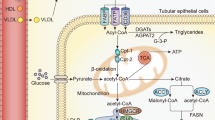
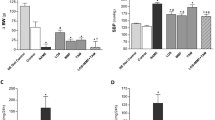
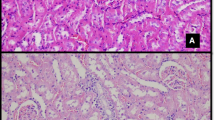
Reducing immunosuppressant-related complications using conventional drugs is an efficient therapeutic strategy. L-carnitine (LC) has been shown to protect against various types of renal injury. In this study, we investigated the renoprotective effects of LC in a rat model of chronic tacrolimus (TAC) nephropathy. SD rats were injected with TAC (1.5 mg · kg−1 · d−1, sc) for 4 weeks. Renoprotective effects of LC were assessed in terms of renal function, histopathology, oxidative stress, expression of inflammatory and fibrotic cytokines, programmed cell death (pyroptosis, apoptosis, and autophagy), mitochondrial function, and PI3K/AKT/PTEN signaling. Chronic TAC nephropathy was characterized by severe renal dysfunction and typical histological features of chronic nephropathy. At a molecular level, TAC markedly increased the expression of inflammatory and fibrotic cytokines in the kidney, induced oxidative stress, and led to mitochondrial dysfunction and programmed cell death through activation of PI3K/AKT and inhibition of PTEN. Coadministration of LC (200 mg · kg−1 · d−1, ip) caused a prominent improvement in renal function and ameliorated histological changes of kidneys in TAC-treated rats. Furthermore, LC exerted anti-inflammatory and antioxidant effects, prevented mitochondrial dysfunction, and modulated the expression of a series of apoptosis- and autophagy-controlling genes to promote cell survival. Human kidney proximal tubular epithelial cells (HK-2 cells) were treated with TAC (50 μg/mL) in vitro, which induced production of intracellular reactive oxygen species and expression of an array of genes controlling programmed cell death (pyroptosis, apoptosis, and autophagy) through interfering with PI3K/AKT/PTEN signaling. The harmful responses of HK-2 cells to TAC were significantly attenuated by cotreatment with LC and the PI3K inhibitor LY294002 (25 μM). In conclusion, LC treatment protects against chronic TAC nephropathy through interfering the PI3K/AKT/PTEN signaling.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bentata Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif Organs. 2020;44:140–52.
Yap DY, Ma MK, Mok MM, Kwan LP, Chan GC, Chan TM. Long-term data on tacrolimus treatment in lupus nephritis. Rheumatology. 2014;53:2232–7.
Sinha A, Sharma A, Mehta A, Gupta R, Gulati A, Hari P, et al. Calcineurin inhibitor induced nephrotoxicity in steroid resistant nephrotic syndrome. Indian J Nephrol. 2013;23:41–6.
Yagisawa T, Omoto K, Shimizu T, Ishida H, Tanabe K. Arteriosclerosis in zero-time biopsy is a risk factor for tacrolimus-induced chronic nephrotoxicity. Nephrology. 2015;20 Suppl 2:51–7.
Lim SW, Shin YJ, Luo K, Quan Y, Ko EJ, Chung BH, et al. Effect of klotho on autophagy clearance in tacrolimus-induced renal injury. FASEB J. 2019;33:2694–706.
Zhang LY, Jin J, Luo K, Piao SG, Zheng HL, Jin JZ, et al. Shen-Kang protects against tacrolimus-induced renal injury. Korean J Intern Med. 2019;34:1078–90.
Ferreira GC, McKenna MC. L-carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem Res. 2017;42:1661–75.
Giudetti AM, Stanca E, Siculella L, Gnoni GV, Damiano F. Nutritional and hormonal regulation of citrate and carnitine/acylcarnitine transporters: two mitochondrial carriers involved in fatty acid metabolism. Int J Mol Sci. 2016;17:E817.
Xue M, Chen X, Guo Z, Liu X, Bi Y, Yin J, et al. L-carnitine attenuates cardiac dysfunction by ischemic insults through Akt signaling pathway. Toxicol Sci. 2017;160:341–50.
Kunak CS, Ugan RA, Cadirci E, Karakus E, Polat B, Un H, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radio. 2016;89:20140724.
Liu Y, Yan S, Ji C, Dai W, Hu W, Zhang W, et al. Metabolomic changes and protective effect of (L)-carnitine in rat kidney ischemia/reperfusion injury. Kidney Blood Press Res. 2012;35:373–81.
Fan JP, Kim D, Kawachi H, Ha TS, Han GD. Ameliorating effects of L-carnitine on diabetic podocyte injury. J Med Food. 2010;13:1324–30.
Zambrano S, Blanca AJ, Ruiz-Armenta MV, Miguel-Carrasco JL, Arevalo M, Mate A, et al. L-carnitine attenuates the development of kidney fibrosis in hypertensive rats by upregulating PPAR-gamma. Am J Hypertens. 2014;27:460–70.
Boonsanit D, Kanchanapangka S, Buranakarl C. L-carnitine ameliorates doxorubicin-induced nephrotic syndrome in rats. Nephrology. 2006;11:313–20.
Xiang Y, Piao SG, Zou HB, Jin J, Fang MR, Lei DM, et al. L-carnitine protects against cyclosporine-induced pancreatic and renal injury in rats. Transplant Proc. 2013;45:3127–34.
Jin J, Jin L, Luo K, Lim SW, Chung BH, Yang CW. Effect of empagliflozin on tacrolimus-induced pancreas islet dysfunction and renal injury. Am J Transplant. 2017;17:2601–16.
Yu JH, Lim SW, Luo K, Cui S, Quan Y, Shin YJ, et al. Coenzyme Q10 alleviates tacrolimus-induced mitochondrial dysfunction in kidney. FASEB J. 2019;33:12288–98.
Lim SW, Shin YJ, Luo K, Quan Y, Cui S, Ko EJ, et al. Ginseng increases klotho expression by FoxO3-mediated manganese superoxide dismutase in a mouse model of tacrolimus-induced renal injury. Aging. 2019;11:5548–69.
Wang S, Xu J, Zheng J, Zhang X, Shao J, Zhao L, et al. Anti-inflammatory and antioxidant effects of acetyl-L-carnitine on atherosclerotic rats. Med Sci Monit. 2020;26:e920250.
Rababa’h SY, Alzoubi KH, Hammad HM, Alquraan L, El-Salem K. Memory impairment induced by chronic psychosocial stress is prevented by L-carnitine. Drug Des Dev Ther. 2019;13:4341–50.
Li M, Xu S, Geng Y, Sun L, Wang R, Yan Y, et al. The protective effects of L-carnitine on myocardial ischaemia-reperfusion injury in patients with rheumatic valvular heart disease undergoing CPB surgery are associated with the suppression of NF-kappaB pathway and the activation of Nrf2 pathway. Clin Exp Pharmacol Physiol. 2019;46:1001–12.
Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, et al. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014;29:1449–57.
Mescka CP, Guerreiro G, Donida B, Marchetti D, Wayhs CA, Ribas GS, et al. Investigation of inflammatory profile in MSUD patients: benefit of L-carnitine supplementation. Metab Brain Dis. 2015;30:1167–74.
Jamilian M, Foroozanfard F, Kavossian E, Aghadavod E, Amirani E, Mahdavinia M, et al. Carnitine and chromium co-supplementation affects mental health, hormonal, inflammatory, genetic, and oxidative stress parameters in women with polycystic ovary syndrome. J Psychosom Obstet Gynaecol. 2019;5:1–9.
Kim YG, Kim SM, Kim KP, Lee SH, Moon JY. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells. 2019;8:E1389.
Liu H, Chen Z, Weng X, Chen H, Du Y, Diao C, et al. Enhancer of zeste homolog 2 modulates oxidative stress-mediated pyroptosis in vitro and in a mouse kidney ischemia-reperfusion injury model. FASEB J. 2020;34:835–52.
Chung SD, Lai TY, Chien CT, Yu HJ. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299.
Peng X, Yang T, Liu G, Liu H, Peng Y, He L. Piperine ameliorated lupus nephritis by targeting AMPK-mediated activation of NLRP3 inflammasome. Int Immunopharmacol. 2018;65:448–57.
Wang Y, Zhu X, Yuan S, Wen S, Liu X, Wang C, et al. TLR4/NF-kappaB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol. 2019;10:603.
Kim SM, Kim YG, Kim DJ, Park SH, Jeong KH, Lee YH, et al. Inflammasome-independent role of NLRP3 mediates mitochondrial regulation in renal injury. Front Immunol. 2018;9:2563.
Shakeri A, Tabibi H, Hedayati M. Effects of L-carnitine supplement on serum inflammatory cytokines, c-reactive protein, lipoprotein (a), and oxidative stress in hemodialysis patients with Lp (a) hyperlipoproteinemia. Hemodial Int. 2010;14:498–504.
Galvan DL, Green NH, Danesh FR. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017;92:1051–7.
Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept. 2010;161:58–66.
Nguyen LT, Stangenberg S, Chen H, Al-Odat I, Chan YL, Gosnell ME, et al. L-carnitine reverses maternal cigarette smoke exposure-induced renal oxidative stress and mitochondrial dysfunction in mouse offspring. Am J Physiol Ren Physiol. 2015;308:F689–96.
Luo K, Lim SW, Jin J, Jin L, Gil HW, Im DS, et al. Cilastatin protects against tacrolimus-induced nephrotoxicity via anti-oxidative and anti-apoptotic properties. BMC Nephrol. 2019;20:221.
Sue YM, Chou HC, Chang CC, Yang NJ, Chou Y, Juan SH. L-carnitine protects against carboplatin-mediated renal injury: AMPK- and PPARα- dependent inactivation of NFAT3. PLoS One. 2014;9:e104079.
Chen W, Ruan R, Zhao S, Ning J, Rao T, Yu W, et al. MicroRNA-205 inhibits the apoptosis of renal tubular epithelial cells via the PTEN/Akt pathway in renal ischemia-reperfusion injury. AmJ Transl Res. 2019;11:7364–75.
Higgins DF, Ewart LM, Masterson E, Tennant S, Grebnev G, Prunotto M, et al. BMP7-induced-Pteninhibits Akt and prevents renal fibrosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:3095–104.
Song Y, Liu W, Tang K, Zang J, Li D, Gao H. Mangiferin alleviates renal interstitial fibrosis in streptozotocin-induced diabetic mice through regulating the PTEN/PI3K/Akt signaling pathway. J Diabetes Res. 2020;2020:9481720.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81560125, 81760293, 81760132, and 81760668).
Author information
Authors and Affiliations
Contributions
CL, CWY, JPL, BHC, and BSC designed the research; YJJ, HLZ, JJ, and SGP performed the research; HYL, HYZ, and CLZ conducted the in vitro study; MYX, YSJ, SC, and JZJ analyzed the data and performed molecular work; and CL wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zheng, Hl., Zhang, Hy., Zhu, Cl. et al. L-Carnitine protects against tacrolimus-induced renal injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Acta Pharmacol Sin 42, 77–87 (2021). https://doi.org/10.1038/s41401-020-0449-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0449-8
Keywords
This article is cited by
-
Comprehensive review of the expanding roles of the carnitine pool in metabolic physiology: beyond fatty acid oxidation
Journal of Translational Medicine (2025)
-
Ferroptosis and pyroptosis are connected through autophagy: a new perspective of overcoming drug resistance
Molecular Cancer (2025)
-
Effect of L-carnitine in Ameliorating Lipopolysaccharide-Induced Cardiomyocyte Injury via MAPK Signaling
Molecular Biotechnology (2024)
-
Linagliptin ameliorates tacrolimus-induced renal injury: role of Nrf2/HO-1 and HIF-1α/CTGF/PAI-1
Molecular Biology Reports (2024)
-
Protective effect of silymarin on tacrolimus-induced kidney and liver toxicity
BMC Complementary Medicine and Therapies (2022)