Abstract
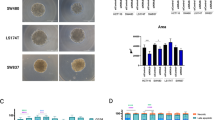
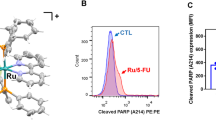
Sphingosine-1-phosphate (S1P), the backbone of most sphingolipids, activating S1P receptors (S1PRs) and the downstream G protein signaling has been implicated in chemoresistance. In this study we investigated the role of S1PR2 internalization in 5-fluorouracil (5-FU) resistance in human colorectal cancer (CRC). Clinical data of randomly selected 60 CRC specimens showed the correlation between S1PR2 internalization and increased intracellular uracil (P < 0.001). Then we explored the regulatory mechanisms in CRC model of villin-S1PR2−/− mice and CRC cell lines. We showed that co-administration of S1P promoted S1PR2 internalization from plasma membrane (PM) to endoplasmic reticulum (ER), thus blunted 5-FU efficacy against colorectal tumors in WT mice, compared to that in S1PR2−/− mice. In HCT116 and HT-29 cells, application of S1P (10 μM) empowered S1PR2 to internalize from PM to ER, thus inducing 5-FU resistance, whereas the specific S1PR2 inhibitor JTE-013 (10 μM) effectively inhibited S1P-induced S1PR2 internalization. Using Mag-Fluo-AM-labeling [Ca2+]ER and LC-ESI-MS/MS, we revealed that internalized S1PR2 triggered elevating [Ca2+]ER levels to activate PERK-eLF2α-ATF4 signaling in HCT116 cells. The activated ATF4 upregulated RNASET2-mediated uracil generation, which impaired exogenous 5-FU uptake to blunt 5-FU therapy. Overall, this study reveals a previously unrecognized mechanism of 5-FU resistance resulted from S1PR2 internalization-upregulated uracil generation in colorectal cancer, and provides the novel insight into the significance of S1PR2 localization in predicting the benefit of CRC patients from 5-FU-based chemotherapy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Soong R, Shah N, Salto-Tellez M, Tai BC, Soo RA, Han HC, et al. Prognostic significance of thymidylate synthase, dihydropyrimidine dehydrogenase and thymidine phosphorylase protein expression in colorectal cancer patients treated with or without 5-fluorouracil-based chemotherapy. Ann Oncol. 2008;19:915–9.
Pare L, Marcuello E, Altes A, Del RE, Sedano L, Salazar J, et al. Pharmacogenetic prediction of clinical outcome in advanced colorectal cancer patients receiving oxaliplatin/5-fluorouracil as first-line chemotherapy. Br J Cancer. 2008;99:1050–5.
Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8.
Furukawa T, Tabata S, Yamamoto M, Kawahara K, Shinsato Y, Minami K, et al. Thymidine phosphorylase in cancer aggressiveness and chemoresistance. Pharmacol Res. 2018;132:15–20.
Crea F, Nobili S, Paolicchi E, Perrone G, Napoli C, Landini I, et al. Epigenetics and chemoresistance in colorectal cancer: an opportunity for treatment tailoring and novel therapeutic strategies. Drug Resist Update. 2011;14:280–96.
Wu R, Nie Q, Tapper EE, Jerde CR, Dunlap GS, Shrestha S, et al. Histone H3K27 trimethylation modulates 5-fluorouracil resistance by inhibiting PU.1 binding to the DPYD promoter. Cancer Res. 2016;76:6362–73.
Marjaneh RM, Khazaei M, Ferns GA, Avan A, Aghaee-Bakhtiari SH. The role of microRNAs in 5-FU resistance of colorectal cancer: possible mechanisms. J Cell Physiol. 2019;234:2306–16.
Lifshitz V, Priceman SJ, Li W, Cherryholmes G, Lee H, Makovski-Silverstein A, et al. Sphingosine-1-phosphate receptor-1 promotes environment-mediated and acquired chemoresistance. Mol Cancer Ther. 2017;16:2516–27.
Guo YX, Ma YJ, Han L, Wang YJ, Han JA, Zhu Y. Role of sphingosine 1-phosphate in human pancreatic cancer cells proliferation and migration. Int J Clin Exp Med. 2015;8:20349–54.
Dempsey LA. Distinct S1PR roles. Nat Immunol. 2019;20:517.
Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50.
Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of the S1P/S1PR axis in health and disease. J Dent Res. 2011;90:841–54.
Stepanovska B, Huwiler A. Targeting the S1P receptor signaling pathways as a promising approach for treatment of autoimmune and inflammatory diseases. Pharmacol Res. 2020;154:104170.
Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–8.
Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–34.
Zhang YH, Luo DD, Wan SB, Qu XJ. S1PR2 inhibitors potently reverse 5-FU resistance by downregulating DPD expression in colorectal cancer. Pharmacol Res. 2020;155:104717.
Ou J, Peng Y, Yang W, Zhang Y, Hao J, Li F, et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat Commun. 2019;10:1078.
Parang B, Barrett CW, Williams CS. AOM/DSS model of colitis-associated cancer. Methods Mol Biol. 2016;1422:297–307.
Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–7.
Wang H, Zhang J, Hu SH, Tan SZ, Zhang B, Zhou HM, et al. Real-time microwave exposure induces calcium efflux in primary hippocampal neurons and primary cardiomyocytes. Biomed Environ Sci. 2018;31:561–71.
Parang B, Barrett CW, Williams CS. AOM/DSS model of colitis-associated cancer. Methods Mol Biol. 2016;1422:297–307.
Kharel Y, Raje M, Gao M, Gellett AM, Tomsig JL, Lynch KR, et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem J. 2012;447:149–57.
Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407.
Lee DK, Min YS, Yoo SS, Shim HS, Park SY, Sohn UD. Effect of sphingosine-1-phosphate on intracellular free Ca2+ in cat esophageal smooth muscle cells. Biomol Ther. 2018;26:546–52.
Villa A, Podini P, Panzeri MC, Soling HD, Volpe P, Meldolesi J. The endoplasmic-sarcoplasmic reticulum of smooth muscle: immunocytochemistry of vas deferens fibers reveals specialized subcompartments differently equipped for the control of Ca2+ homeostasis. J Cell Biol. 1993;121:1041–51.
Auberger P, Puissant A. Autophagy, a key mechanism of oncogenesis and resistance in leukemia. Blood. 2017;129:547–52.
Wu HB, Yang S, Weng HY, Chen Q, Zhao XL, Fu WJ, et al. Autophagy-induced KDR/VEGFR-2 activation promotes the formation of vasculogenic mimicry by glioma stem cells. Autophagy. 2017;13:1528–42.
Wu QB, Sheng X, Zhang N, Yang MW, Wang F. Role of microRNAs in the resistance of colorectal cancer to chemoradiotherapy. Mol Clin Oncol. 2018;8:528–32.
Jeon SJ, Ahn JH, Halder D, Cho HS, Lim JH, Jun SY, et al. TIPRL potentiates survival of lung cancer by inducing autophagy through the eIF2alpha-ATF4 pathway. Cell Death Dis. 2019;10:959.
Thorn A, Steinfeld R, Ziegenbein M, Grapp M, Hsiao HH, Urlaub H, et al. Structure and activity of the only human RNase T2. Nucleic Acids Res. 2012;40:8733–42.
Haud N, Kara F, Diekmann S, Henneke M, Willer JR, Hillwig MS, et al. rnaset2 mutant zebrafish model familial cystic leukoencephalopathy and reveal a role for RNase T2 in degrading ribosomal RNA. Proc Natl Acad Sci USA. 2011;108:1099–103.
Antoon JW, White MD, Slaughter EM, Driver JL, Khalili HS, Elliott S, et al. Targeting NFkB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol Ther. 2011;11:678–89.
Mathes C, Fleig A, Penner R. Calcium release-activated calcium current (ICRAC) is a direct target for sphingosine. J Biol Chem. 1998;273:25020–30.
Meyer ZHD. Lysophospholipid receptor-dependent and -independent calcium signaling. J Cell Biochem. 2004;92:937–48.
Melendez AJ. Sphingosine kinase signalling in immune cells: potential as novel therapeutic targets. Biochim Biophys Acta. 2008;1784:66–75.
Reuschlein AK, Jakobsen E, Mertz C, Bak LK. Aspects of astrocytic cAMP signaling with an emphasis on the putative power of compartmentalized signals in health and disease. Glia. 2019;67:1625–36.
Halls ML, Cooper DM. Regulation by Ca2+-signaling pathways of adenylyl cyclases. Cold Spring Harb Perspect Biol. 2011;3:a4143.
Michalakis S, Becirovic E, Biel M. Retinal cyclic nucleotide-gated channels: from pathophysiology to therapy. Int J Mol Sci. 2018;19:749.
Straub SV, Wagner LN, Bruce JI, Yule DI. Modulation of cytosolic calcium signaling by protein kinase A-mediated phosphorylation of inositol 1,4,5-trisphosphate receptors. Biol Res. 2004;37:593–602.
Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–52.
Luhtala N, Parker R. T2 Family ribonucleases: ancient enzymes with diverse roles. Trends Biochem Sci. 2010;35:253–9.
Suzuki A, Yao M, Tanaka I, Numata T, Kikukawa S, Yamasaki N, et al. Crystal structures of the ribonuclease MC1 from bitter gourd seeds, complexed with 2’-UMP or 3’-UMP, reveal structural basis for uridine specificity. Biochem Biophys Res Commun. 2000;275:572–6.
Van Triest B, Pinedo HM, Giaccone G, Peters GJ. Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann Oncol. 2000;11:385–91.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91629303/81673449/81872884/81973350) and the Beijing Natural Science Foundation and Scientific Research Program of Municipal Commission of Education (KZ201710025020/KZ201810025033). In addition, YHZ wants to thank, in particular, the inimitable support and care from Wen-yu Wang. Hope to spend the rest of my life with you.
Author information
Authors and Affiliations
Contributions
XJQ conceived the project. YHZ performed the experiments of molecular mechanisms and conducted animal studies. SBW implemented experiments of pharmaceutical analysis. SHW provided clinical samples and pathological analysis. SXC performed statistical analysis. YHZ wrote the manuscript, which was edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, Yh., Cui, Sx., Wan, Sb. et al. Increased S1P induces S1PR2 internalization to blunt the sensitivity of colorectal cancer to 5-fluorouracil via promoting intracellular uracil generation. Acta Pharmacol Sin 42, 460–469 (2021). https://doi.org/10.1038/s41401-020-0460-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0460-0
Keywords
This article is cited by
-
Importance of PERK pathway modulation on colorectal cancer management: a systematic review
BMC Cancer (2025)
-
Magnetic fabric from Red clay sediments in the Chinese Loess Plateau
Scientific Reports (2015)