Abstract
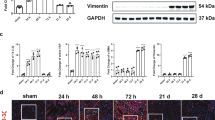
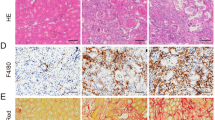
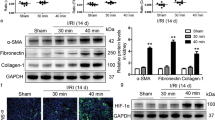
Acute renal injury (AKI) causes a long-term risk for progressing into chronic kidney disease (CKD) and interstitial fibrosis. Yes-associated protein (YAP), a key transcriptional cofactor in Hippo signaling pathway, shuttles between the cytoplasm and nucleus, which is required for the renal tubular epithelial cells repair in the acute phase of AKI. In this study we investigated the role of YAP during ischemia-reperfusion (IR)-induced AKI to CKD. Mice were subjected to left kidney IR followed by removal of the right kidney on the day before tissue harvests. Mouse shRNA expression adenovirus (Ad-shYAP or Ad-shKLF4) and mouse KLF4 expression adenovirus (Ad-KLF4) were delivered to mice by intrarenal injection on D7 after IR. We showed that the expression and nucleus distribution of YAP were persistently increased until the end of experiment (D21 after IR). The sustained activation of YAP in post-acute phase of AKI was accompanied by renal dysfunction and interstitial fibrosis. Knockdown of YAP significantly attenuated IR-induced renal dysfunction and decreased the expression of fibrogenic factors TGF-β and CTGF in the kidney. We showed that the expression of the transcription factor KLF4, lined on the upstream of YAP, was also persistently increased. Knockdown on KLF4 attenuated YAP increase and nuclear translocation as well as renal functional deterioration and interstitial fibrosis in IR mice, whereas KLF4 overexpression caused opposite effects. KLF4 increased the expression of ITCH, and ITCH facilitated YAP nuclear translocation via degrading LATS1. Furthermore, we demonstrated in primary cultured renal tubular cells that KLF4 bound to the promoter region of YAP and positively regulates YAP expression. In biopsy sample from CKD patients, we also observed increased expression and nuclear distribution of YAP. In conclusion, the activation of YAP in the post-acute phase of AKI is implicated in renal functional deterioration and fibrosis although it exhibits beneficial effect in acute phase. Reprogramming factor KLF4 is responsible for the persistent activation of YAP. Blocking the activation of KLF4-YAP pathway might be a way to prevent the transition of AKI into CKD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vanmassenhove J, Van Biesen W, Vanholder R, Lameire N. Subclinical AKI: ready for primetime in clinical practice? J Nephrol. 2019;32:9–16.
Parikh CR, Mansour SG. Perspective on clinical application of biomarkers in AKI. J Am Soc Nephrol. 2017;28:1677–85.
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–72.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8.
Xu D, Wang B, Chen P, Wang YZ, Miao NJ, Yin F, et al. c-Myc promotes tubular cell apoptosis in ischemia-reperfusion-induced renal injury by negatively regulating c-FLIP and enhancing FasL/Fas-mediated apoptosis pathway. Acta Pharmacol Sin. 2019;40:1058–66.
Srisawat N, Murugan R, Kellum JA. Repair or progression after AKI: a role for biomarkers? Nephron Clin Pr. 2014;127:185–89.
Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–76.
Chatauret N, Badet L, Barrou B, Hauet T. Ischemia-reperfusion: from cell biology to acute kidney injury. Prog Urol. 2014;24(Suppl 1):S4–12.
Bonventre JV. Primary proximal tubule injury leads to epithelial cell cycle arrest, fibrosis, vascular rarefaction, and glomerulosclerosis. Kidney Int Suppl (2011). 2014;4:39–44.
Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Ren Physiol. 2014;307:F1187–95.
Bonventre JV. Maladaptive proximal tubule repair: cell cycle arrest. Nephron Clin Pract. 2014;127:61–4.
Goldstein SL, Jaber BL, Faubel S, Chawla LS. AKI transition of care: a potential opportunity to detect and prevent CKD. Clin J Am Soc Nephrol. 2013;8:476–83.
Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27:687–97.
Humphreys BD, Cantaluppi V, Portilla D, Singbartl K, Yang L, Rosner MH, et al. Targeting endogenous repair pathways after AKI. J Am Soc Nephrol. 2016;27:990–8.
Maas K, Mirabal S, Penzias A, Sweetnam PM, Eggan KC, Sakkas D. Hippo signaling in the ovary and polycystic ovarian syndrome. J Assist Reprod Genet. 2018;35:1763–71.
Frum T, Murphy TM, Ralston A. HIPPO signaling resolves embryonic cell fate conflicts during establishment of pluripotency in vivo. Elife. 2018;7:e42298. https://doi.org/10.7554/eLife.42298.
5Volckaert T, Yuan T, Yuan J, Boateng E, Hopkins S, Zhang JS, et al. Hippo signaling promotes lung epithelial lineage commitment by curbing Fgf10 and beta-catenin signaling. Development. 2019;146:dev166454. https://doi.org/10.1242/dev.166454.
Nair PR, Wirtz D. Enabling migration by moderation: YAP/TAZ are essential for persistent migration. J Cell Biol. 2019;218:1092–3.
Han H, Yang B, Nakaoka HJ, Yang J, Zhao Y, Le Nguyen K, et al. Hippo signaling dysfunction induces cancer cell addiction to YAP. Oncogene. 2018;37:6414–24.
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18.
White SM, Murakami S, Yi C. The complex entanglement of Hippo-Yap/Taz signaling in tumor immunity. Oncogene. 2019;38:2899–909.
Sugihara T, Isomoto H, Gores G, Smoot R. YAP and the hippo pathway in cholangiocarcinoma. J Gastroenterol. 2019;54:485–91.
Chen J, You H, Li Y, Xu Y, He Q, Harris RC. EGF receptor-dependent YAP activation is important for renal recovery from AKI. J Am Soc Nephrol. 2018;29:2372–85.
Sharma M, Radhakrishnan R. CTGF is obligatory for TGF-beta1 mediated fibrosis in OSMF. Oral Oncol. 2016;56:e10–11.
Li L, Dong L, Wang Y, Zhang X, Yan J. Lats1/2-mediated alteration of hippo signaling pathway regulates the fate of bone marrow-derived mesenchymal stem cells. Biomed Res Int. 2018;2018:4387932.
Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, et al. The hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410.
Ho KC, Zhou Z, She YM, Chun A, Cyr TD, Yang X. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability. Proc Natl Acad Sci USA. 2011;108:4870–5.
Marzi I, Cipolleschi MG, D’Amico M, Stivarou T, Rovida E, Vinci MC, et al. The involvement of a Nanog, Klf4 and c-Myc transcriptional circuitry in the intertwining between neoplastic progression and reprogramming. Cell Cycle. 2013;12:353–64.
Choi H, Roh J. Role of klf4 in the regulation of apoptosis and cell cycle in rat granulosa cells during the periovulatory period. Int J Mol Sci. 2018;20:87. https://doi.org/10.3390/ijms20010087.
Tang J, Zhong G, Wu J, Chen H, Jia Y. SOX2 recruits KLF4 to regulate nasopharyngeal carcinoma proliferation via PI3K/AKT signaling. Oncogenesis. 2018;7:61. https://doi.org/10.1038/s41389-018-0074-2.
Cheng Z, Zou X, Jin Y, Gao S, Lv J, Li B, et al. The role of KLF4 in Alzheimer's disease. Front Cell Neurosci. 2018;12:325. https://doi.org/10.3389/fncel.2018.00325.
Brauer PR, Kim JH, Ochoa HJ, Stratton ER, Black KM, Rosencrans W, et al. Kruppel-like factor 4 mediates cellular migration and invasion by altering RhoA activity. Cell Commun Adhes. 2018;24:1–10.
Qi XT, Li YL, Zhang YQ, Xu T, Lu B, Fang L, et al. KLF4 functions as an oncogene in promoting cancer stem cell-like characteristics in osteosarcoma cells. Acta Pharmacol Sin. 2019;40:546–55.
Shen Y, Miao N, Wang B, Xu J, Gan X, Xu D, et al. c-Myc promotes renal fibrosis by inducing integrin alphav-mediated transforming growth factor-beta signaling. Kidney Int. 2017;92:888–99.
Pan Y, Alegot H, Rauskolb C, Irvine KD. The dynamics of Hippo signaling during Drosophila wing development. Development. 2018;145:dev165712. https://doi.org/10.1242/dev.165712.
Xu D, Chen P, Wang B, Wang Y, Miao N, Yin F, et al. NIX-mediated mitophagy protects against proteinuria-induced tubular cell apoptosis and renal injury. Am J Physiol Renal Physiol. 2019;316:F382–95.
Bernhardt A, Fehr A, Brandt S, Jerchel S, Ballhause TM, Philipsen L, et al. Inflammatory cell infiltration and resolution of kidney inflammation is orchestrated by the cold-shock protein Y-box binding protein-1. Kidney Int. 2017;92:1157–77.
Fu Y, Tang C, Cai J, Chen G, Zhang D, Dong Z. Rodent models of AKI-CKD transition. Am J Physiol-Ren. 2018;315:F1098–106.
Luo C, Zhou S, Zhou Z, Liu Y, Yang L, Liu J, et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J Am Soc Nephrol. 2018;29:1238–56.
Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–79.
Tan RJ, Zhou D, Liu Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis. 2016;2:136–44.
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, et al. YAP/TAZ are mechanoregulators of TGF-beta-Smad signaling and renal fibrogenesis. ed. 2016:3117–28.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96.
Macconi D, Remuzzi G, Benigni A. Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int Suppl (2011). 2014;4:58–64.
Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–61.
Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70:1914–9.
Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol. 2014;25:2187–200.
Ding H, Zhou D, Hao S, Zhou L, He W, Nie J, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–13.
Yoshida T, Yamashita M, Iwai M, Hayashi M. Endothelial Kruppel-Like factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol. 2016;27:1379–88.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China, No. 81873603 and 81670664 to LML and No. 81770718 to XXW. This work was also supported by the Science and Technology Commission of Shanghai Municipality (14DZ2260200, the project of Shanghai Key Laboratory of Kidney and BLood Purification).
Author information
Authors and Affiliations
Contributions
DX, LML, and WZ participated in the design of the study. DX wrote the paper. DX and PPC were involved in performing the experiments and analyzing the results. DX, PPC, PQZ, FY, QC, ZLZ, HYX, JYL, JYN, YZW, SJC, LZ, XXW, and JL took part in the revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Xu, D., Chen, Pp., Zheng, Pq. et al. KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharmacol Sin 42, 436–450 (2021). https://doi.org/10.1038/s41401-020-0463-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0463-x
Keywords
This article is cited by
-
iPSC-based drug discovery identified the Hippo signaling pathway as a therapeutic target in the fibrosis of NPHP1-deficient nephronophthisis
Stem Cell Research & Therapy (2025)
-
USP11 promotes renal tubular cell pyroptosis and fibrosis in UUO mice via inhibiting KLF4 ubiquitin degradation
Acta Pharmacologica Sinica (2025)
-
Dynamics of chromatin accessibility governing Gd-IgA1 synthesis in B cells associated with IgA nephropathy
Experimental & Molecular Medicine (2025)
-
Role of biophysics and mechanobiology in podocyte physiology
Nature Reviews Nephrology (2024)
-
N6-methyladenosine methylation in kidney injury
Clinical Epigenetics (2023)