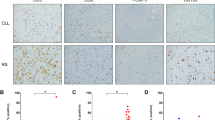
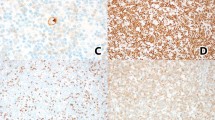
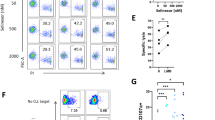
Abstract
Cutaneous T-cell lymphoma (CTCL) is characterized by a heterogeneous group of extranodal non-Hodgkin lymphomas, in which monoclonal T lymphocytes infiltrate the skin. LW-213, a derivative of wogonin, was found to induce cell apoptosis in chronic myeloid leukemia (CML). In this study, we investigated the effects of LW-213 on CTCL cells and the underlying mechanisms. We showed that LW-213 (1–25 μM) dose-dependently inhibited human CTCL cell lines (Hut-102, Hut-78, MyLa, and HH) with IC50 values of around 10 μM, meanwhile it potently inhibited primary leukemia cells derived from peripheral blood of T-cell lymphoma patients. We revealed that LW-213-induced apoptosis was accompanied by ROS formation and the release of calcium from endoplasmic reticulum (ER) through IP3R-1channel. LW-213 selectively activated CHOP and induced apoptosis in Hut-102 cells via activating PERK–eIF2α–ATF4 pathway. Interestingly, the degree of apoptosis and expression of ER stress-related proteins were alleviated in the presence of either N-acetyl cysteine (NAC), an ROS scavenger, or 2-aminoethyl diphenylborinate (2-APB), an IP3R-1 inhibitor, implicating ROS/calcium-dependent ER stress in LW-213-induced apoptosis. In NOD/SCID mice bearing Hut-102 cell line xenografts, administration of LW-213 (10 mg/kg, ip, every other day for 4 weeks) markedly inhibited the growth of Hut-102 derived xenografts and prolonged survival. In conclusion, our study provides a new insight into the mechanism of LW-213-induced apoptosis, suggesting the potential of LW-213 as a promising agent against CTCL.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Khadhar A, Chelly I, Zehani A, Litaiem N, Zaraa I, Azouz H, et al. A challenging cutaneous T-cell lymphoma. Am J Dermatopathol. 2016;38:63–5.
Ryan A. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017;92:1085–102.
Quaglino P, Maule M, Prince HM, Porcu P, Horwitz S, Duvic M, et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol. 2017;28:2517–25.
Bagherania N, Bruce R. An overview of cutaneous T cell lymphomas. F1000Res. 2016;5:F1000.
Sam T, John E, Elaine S, Wyndham H. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945–57.
Ryan A. Hematology, cutaneous T-cell lymphoma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:837–51.
Verfaillie T, Abhishek D, Patrizia A. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013;332:249–64.
Maurel M, Eoghan P, Mnich K, Healy S, Chevet E, Samali A. Controlling the unfolded protein response-mediated life and death decisions in cancer. Semin Cancer Biol. 2015;33:57–66.
Mori K. The unfolded protein response: the dawn of a new field. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91:469–80.
Wang M, Randal J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–97.
Madden E, Susan E, Sandra J, Manie S, Samali A. The role of the unfolded protein response in cancer progression: from oncogenesis to chemoresistance. Biol Cell. 2019;111:1–17.
Ching-Fen W, JeongSeo E, Sabine M, Klauck, Efferth T. Cryptotanshinone deregulates unfolded protein response and eukaryotic initiation factor signaling in acute lymphoblastic leukemia cells. Phytomedicine. 2016;23:174–80.
Cosenza M, Civallero M, Fiorcari S, Pozzi S, Marcheselli L, Bari A, et al. The histone deacetylase inhibitor romidepsin synergizes with lenalidomide and enhances tumor cell death in T-cell lymphoma cell lines. Cancer Biol Ther. 2016;17:1094–106.
Mengxiong W, Mary E, Ronald K, Brian K. The unfolded protein response as a target for anticancer therapeutics. Crit Rev Oncol Hematol. 2018;127:66–79.
Verfaillie T, Rubio N, Garg A, Bultynck G, Rizzuto R, Decuypere J, et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–91.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl J, Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16:533–44.
Zong ZH, Du ZX, Li N, Li C, Zhang Q, Liu BQ, et al. Implication of Nrf2 and ATF4 in differential induction of CHOP by proteasome inhibition in thyroid cancer cells. Biochim Biophys Acta. 2012;1823:1395–404.
Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25:563–73.
Cabrera E, Hernández-Pérez S, Koundrioukoff S, Debatisse M, Kim DB, Smolka M, et al. PERK inhibits DNA replication during the unfolded protein response via claspin and Chk1. Oncogene. 2017;36:678–86.
Singh M, Uman S, Shukla Y. New enlightenment of skin cancer chemoprevention through phytochemicals: in vitro and in vivo studies and the underlying mechanisms. Biomed Res Int. 2014;2014:2434–52. https://doi.org/10.1155/2014/243452.
Mao XY, Jin MZ, Chen JF, Zhou HH, Jin WL. Live or let die: neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol Ther. 2018;183:137–51.
Huang AC, Chang CL, Yu CS, Chen PY, Yang JS, Ji BC, et al. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI-3 cells is mediated via endoplasmic reticulum stress and mitochondria-dependent pathways. Environ Toxicol. 2013;28:255–66.
Heo JR, Kim SM, Hwang KA, Kang JH, Choi KC. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress‑mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int J Mol Med. 2018;42:1427–35.
Davalli P, Rizzi F, Caldara G, Davoli S, Corti A, Silva A, et al. Chronic administration of green tea extract to TRAMP mice induces the collapse of Golgi apparatus in prostate secretory cells and results in alterations of protein post-translational processing. Int J Oncol. 2011;39:1521–7.
Gongbo L, Sakina M, Nonn L, Jeremy J. Inhibition of CHOP accentuates the apoptotic effect of α-mangostin from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate cancer cells. Biochem Biophys Res Commun. 2014;453:75–80.
Huang X, Li L, Zhang L, Zhang Z, Wang X, Zhang X, et al. Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. Br J Nutr. 2013;109:727–35.
Zhao L, Miao HC, Li WJ, Sun Y, Huang SL, Li ZY, et al. LW-213 induces G2/M cell cycle arrest through AKT/GSK3β/β-catenin signaling pathway in human breast cancer cells. Mol Carcinog. 2016;55:778–92.
Liu X, Hu P, Li H, Yu XX, Wang XY, Qing YJ, et al. LW-213, a newly synthesized flavonoid, induces G2/M phase arrest and apoptosis in chronic myeloid leukemia. Acta Pharmacol Sin. 2020;41:249–59.
Ping J, Li JT, Liao ZX, Shang L, Wang H. Indole-3-carbinol inhibits hepatic stellate cells proliferation by blocking NADPH oxidase/reactive oxygen species/p38 MAPK pathway. Eur J Pharmacol. 2011;650:656.
Li H, Yu X, Liu X, Hu P, Shen L, Zhou Y, et al. Wogonoside induces depalmitoylation and translocation of PLSCR1 and N-RAS in primary acute myeloid leukaemia cells. J Cell Mol Med. 2018;22:2117–30.
Cotter T. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–7.
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–18.
Krebs J, Agellon L, Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem Biophys Res Commun. 2015;460:114–21.
Yamada N, Makino Y, Clark R, Pearson D, Mattei M, Guénet J, et al. Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem J. 1994;302:781–90.
Logue S, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–46.
Mengxiong W, Mary E, Castellano R, Brian K. The unfolded protein response as a target for anticancer therapeutics. Crit Rev Oncol Hematol. 2018;127:66–79.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl J, Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16:533–44.
Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9.
Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9.
Thomas G. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–7.
Portt L, Norman G, Clapp C, Greenwood M, Greenwood M. Anti-apoptosis and cell survival: a review. Biochim Biophys Acta. 2011;1813:238–59.
Shetty S, Gladden J, Henson E, Hu X, Villanueva J, Haney N, et al. Tumor necrosis factor-related apoptosis inducing ligand (TRAIL) up-regulates death receptor 5 (DR5) mediated by NFκB activation in epithelial derived cell lines. Apoptosis. 2002;7:413–20.
Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502.
Singletary K. Diet, natural products and cancer chemoprevention. J Nutr. 2000;130:465S–6S.
Wu R, Murali R, Kabe Y, French SW, Chiang YM, Liu S, et al. Baicalein targets GTPase‐mediated autophagy to eliminate liver tumor–initiating stem cell–like cells resistant to mTORC1 inhibition. Hepatology. 2018;68:1726–40.
Dürr C, Hanna B, Schulz A, Lucas F, Zucknick M, Benner A, et al. TNF recetor signaling is a driver of chronic lymphocytic leukemia that can be therapeutically targeted by the flavonoid wogonin. Haematologica. 2018;103:688–97.
Litao Z, Li Z, Hu W, Yu W, Di P, Jing Y, et al. Oroxylin A reverses P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by G2/M arrest. Toxicol Lett. 2013;219:107–15.
Baumann S, Stefanie C, Giaisi M, Müller W, Merling A, Gülow K, et al. Wogonin preferentially kills malignant lymphocytes and suppresses T-cell tumor growth by inducing PLCgamma1- and Ca2+-depend ent apoptosis. Blood. 2008;111:2354–63.
Kohanski M, Dwyer D, Hayete B, Lawrence C, Collins J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810.
Bhandary B, Marahatta A, Kim H, Chae H. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int J Mol Sci. 2012;14:434–56.
Zhou Y, Shu F, Liang X, Chang H, Shi L, Peng X, et al. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS One. 2014;9:e89021.
Choi A, Hyun J, Hwang K, Jeong Y, Choe W, Yoon K, et al. Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1−, Ca2+-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis. 2014;19:682–97.
Yang L, Wang Q, Li D, Zhou Y, Zheng X, Sun H, et al. Wogonin enhances antitumor activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo through ROS-mediated downregulation of cFLIPLand IAP proteins. Apoptosis. 2013;18:618–26.
Tsai CF, Yeh WL, Huang SM, Tan TW, Lu DY. Wogonin induces reactive oxygen species production and cell apoptosis in human glioma cancer cells. Int J Mol Sci. 2012;13:9877–92.
Giri D, Aggarwal B. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells: autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14.
Bubici C, Papa S, Pham C, Zazzeroni F, Franzoso G. The NF-kappaB-mediated control of ROS and JNK signaling. Histol Histopathol. 2006;21:69–80.
Schröder M, Randal J. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63.
Sano R, Reed J. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–70.
Liang SH, Zhang W, McGrath BC, Zhang P, Cavener DR. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem J. 2006;393:201–9.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81873046, 81830105, 81903647, 81503096, and 81673461), the Drug Innovation Major Project (2017ZX09301014, 2018ZX09711001-003-007, and 2017ZX09101003-005-023), Natural Science Foundation of Jiangsu Province (BK20190560 and BE2018711), Nanjing Medical Science and Technology Development Project (YKK17074), Research and Innovation Project for College Graduates of Jiangsu Province (KYCX18_0803), China Postdoctoral Science Foundation (No. 2018M642373), and “Double First-Class” University project (CPU 2018GF11 and CPU2018GF05).
Author information
Authors and Affiliations
Contributions
XXY designed and performed research, analyzed data, and wrote the paper; HL and MYZ performed research and analyzed data; PH and JRW performed research; YJQ and XYW collected data and performed statistical analysis; HZW and ZYW collected and analyzed data; JYX provided the blood samples; and QG and HH conceptualized the project and directed the experimental design and data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yu, Xx., Zhu, My., Wang, Jr. et al. LW-213 induces cell apoptosis in human cutaneous T-cell lymphomas by activating PERK–eIF2α–ATF4–CHOP axis. Acta Pharmacol Sin 42, 290–300 (2021). https://doi.org/10.1038/s41401-020-0466-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41401-020-0466-7
Keywords
This article is cited by
-
Eukaryotic initiation factor 3a promotes the development of diffuse large B-cell lymphoma through regulating cell proliferation
BMC Cancer (2024)
-
Inhibition of SRPK1, a key splicing regulator, exhibits antitumor and chemotherapeutic-sensitizing effects on extranodal NK/T-cell lymphoma cells
BMC Cancer (2022)